Back
Poster Session E - Tuesday Afternoon
E0360 - Model-Predicted Lymphocyte Response and Recovery Profiles for the Sphingosine 1-Phosphate Receptor Modulators Ozanimod and Etrasimod
Tuesday, October 25, 2022
3:00 PM – 5:00 PM ET
Location: Crown Ballroom
.jpg)
Caroline A. Lee, PhD
Arena Pharmaceuticals, a wholly-owned subsidiary of Pfizer Inc.
San Diego, CA
Presenting Author(s)
Caroline A. Lee, PhD1, Timothy Waterhouse, PhD2, Michael Heathman, MS2, D. Alex Oh, PhD1, H. Kiyomi Komori, PhD1, John S. Grundy, PhD1
1Arena Pharmaceuticals, a wholly-owned subsidiary of Pfizer Inc., San Diego, CA; 2Metrum Research Group, Tariffville, CT
Introduction: Sphingosine 1-phosphate (S1P) receptor modulators, including ozanimod (OZA) and etrasimod (ETR), reversibly sequester lymphocyte egress from lymph nodes, thereby reducing the number of peripheral circulating lymphocytes and their subsequent recruitment to sites of inflammation. Exposure-response models describing observed/published pharmacokinetic (PK) and pharmacodynamic (PD) data can be useful tools for multiple purposes including facilitating comparisons between compounds. Here we present model-predicted lymphocyte response profiles for once-daily dosing of OZA and ETR as well as lymphocyte recovery profiles following drug discontinuation.
Methods: A population PK/PD model of lymphocyte response to OZA over time was developed from published summary-level data for CC112273 PK profile and an OZA lymphocyte Emax model. Initial model simulation predicted a mean baseline lymphocyte count of 1.97x109/L (slightly higher than the observed mean baseline in the OZA RADIANCE study [1.83x109/L]); thus, a correction factor of 0.93 was applied to subsequent OZA simulations. A population PK/PD model of lymphocyte response to ETR over time was developed using data from 7 Phase 1 studies in healthy volunteers and 2 Phase 2 studies in participants with either ulcerative colitis or atopic dermatitis. Simulations from both models of 10,000 virtual participants given OZA (initial 7-day dose titration: 0.23mg on Days 1–4, 0.46mg on Days 5–7, 0.92mg on Day 8 and thereafter for 11 weeks) or ETR (2mg for 12 weeks) were produced and compared.
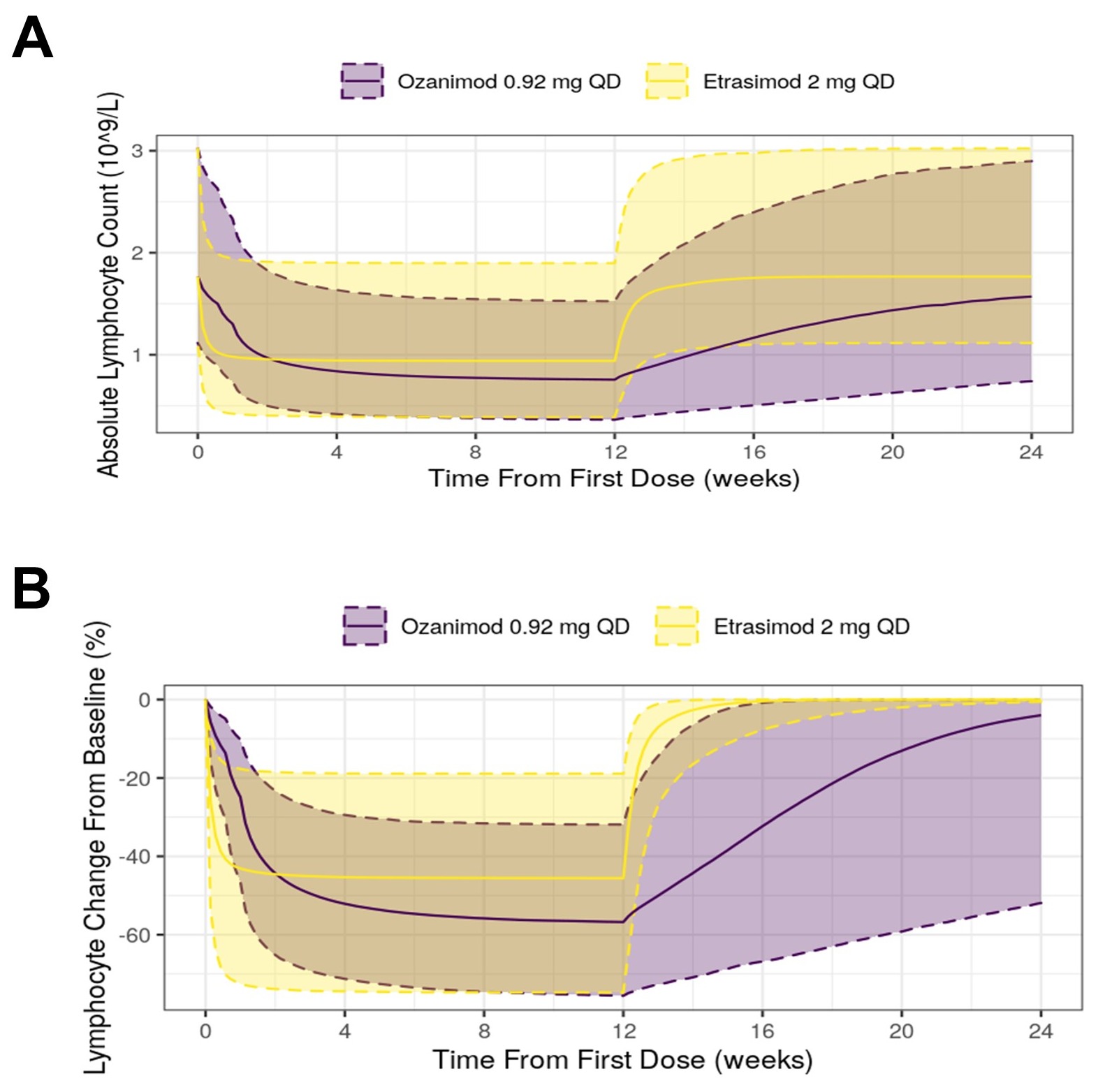
Results: Model-predicted lymphocyte response over time approximately (≥90%) reached median steady-state lymphocyte nadir within 24 days for OZA vs within 5 days for ETR (Figure 1). After drug discontinuation, the predicted time for lymphocyte counts to return to the lower end of the normal range (of either 0.8x109/L or 1.0x109/L for 90% of virtual participants) was 48 and 81 days, respectively, for OZA and 2 and 4 days, respectively, for ETR. The model predicted results for OZA appear generally consistent with similar observed lymphocyte findings reported in its drug label.
Discussion: Model predictions of lymphocyte response and recovery profiles indicate that ETR, when compared to OZA, is expected to achieve lymphocyte nadir more quickly as well as require less time to recover to the normal range after drug discontinuation.
Disclosures:
Caroline A. Lee, PhD1, Timothy Waterhouse, PhD2, Michael Heathman, MS2, D. Alex Oh, PhD1, H. Kiyomi Komori, PhD1, John S. Grundy, PhD1. E0360 - Model-Predicted Lymphocyte Response and Recovery Profiles for the Sphingosine 1-Phosphate Receptor Modulators Ozanimod and Etrasimod, ACG 2022 Annual Scientific Meeting Abstracts. Charlotte, NC: American College of Gastroenterology.
1Arena Pharmaceuticals, a wholly-owned subsidiary of Pfizer Inc., San Diego, CA; 2Metrum Research Group, Tariffville, CT
Introduction: Sphingosine 1-phosphate (S1P) receptor modulators, including ozanimod (OZA) and etrasimod (ETR), reversibly sequester lymphocyte egress from lymph nodes, thereby reducing the number of peripheral circulating lymphocytes and their subsequent recruitment to sites of inflammation. Exposure-response models describing observed/published pharmacokinetic (PK) and pharmacodynamic (PD) data can be useful tools for multiple purposes including facilitating comparisons between compounds. Here we present model-predicted lymphocyte response profiles for once-daily dosing of OZA and ETR as well as lymphocyte recovery profiles following drug discontinuation.
Methods: A population PK/PD model of lymphocyte response to OZA over time was developed from published summary-level data for CC112273 PK profile and an OZA lymphocyte Emax model. Initial model simulation predicted a mean baseline lymphocyte count of 1.97x109/L (slightly higher than the observed mean baseline in the OZA RADIANCE study [1.83x109/L]); thus, a correction factor of 0.93 was applied to subsequent OZA simulations. A population PK/PD model of lymphocyte response to ETR over time was developed using data from 7 Phase 1 studies in healthy volunteers and 2 Phase 2 studies in participants with either ulcerative colitis or atopic dermatitis. Simulations from both models of 10,000 virtual participants given OZA (initial 7-day dose titration: 0.23mg on Days 1–4, 0.46mg on Days 5–7, 0.92mg on Day 8 and thereafter for 11 weeks) or ETR (2mg for 12 weeks) were produced and compared.
Results: Model-predicted lymphocyte response over time approximately (≥90%) reached median steady-state lymphocyte nadir within 24 days for OZA vs within 5 days for ETR (Figure 1). After drug discontinuation, the predicted time for lymphocyte counts to return to the lower end of the normal range (of either 0.8x109/L or 1.0x109/L for 90% of virtual participants) was 48 and 81 days, respectively, for OZA and 2 and 4 days, respectively, for ETR. The model predicted results for OZA appear generally consistent with similar observed lymphocyte findings reported in its drug label.
Discussion: Model predictions of lymphocyte response and recovery profiles indicate that ETR, when compared to OZA, is expected to achieve lymphocyte nadir more quickly as well as require less time to recover to the normal range after drug discontinuation.

Figure: Figure 1. Absolute Lymphocyte Count (A) and Percent Change From Baseline (B) vs Time Profiles for Ozanimod and Etrasimod
Disclosures:
Caroline Lee: Arena Pharmaceuticals, a wholly-owned subsidiary of Pfizer Inc – Employee.
Timothy Waterhouse: Metrum Research Group, which received funding from Arena Pharmaceuticals for conduct of the reported analyses – Employee.
Michael Heathman: Metrum Research Group, which received funding from Arena Pharmaceuticals for conduct of the reported analyses – Employee.
D. Alex Oh: Arena Pharmaceuticals, a wholly-owned subsidiary of Pfizer Inc – Employee.
H. Kiyomi Komori: Arena Pharmaceuticals, a wholly-owned subsidiary of Pfizer Inc – Employee. Arena Pharmaceuticals, a wholly-owned subsidiary of Pfizer Inc – Employee.
John Grundy: Arena Pharmaceuticals, a wholly-owned subsidiary of Pfizer Inc – Employee. Arena Pharmaceuticals, a wholly-owned subsidiary of Pfizer Inc – Employee. Arena Pharmaceuticals, a wholly-owned subsidiary of Pfizer Inc – Employee.
Caroline A. Lee, PhD1, Timothy Waterhouse, PhD2, Michael Heathman, MS2, D. Alex Oh, PhD1, H. Kiyomi Komori, PhD1, John S. Grundy, PhD1. E0360 - Model-Predicted Lymphocyte Response and Recovery Profiles for the Sphingosine 1-Phosphate Receptor Modulators Ozanimod and Etrasimod, ACG 2022 Annual Scientific Meeting Abstracts. Charlotte, NC: American College of Gastroenterology.
