Back
Poster Session C - Monday Afternoon
C0390 - Safety and Efficacy of Vedolizumab in Elderly Patients With Inflammatory Bowel Disease: A Systematic Review and Meta-Analysis
Monday, October 24, 2022
3:00 PM – 5:00 PM ET
Location: Crown Ballroom

Dushyant S. Dahiya, MD
Central Michigan University College of Medicine
Saginaw, MI
Presenting Author(s)
Dushyant S. Dahiya, MD1, Saurabh Chandan, MD2, Jay Bapaye, MD3, Babu P. Mohan, MD, MS4, Daryl Ramai, MD, MPH4, Lena L. Kassab, MD5, Ojasvini C. Chandan, MD6, Parambir S. Dulai, MD7, Gursimran S. Kochhar, MD8
1Central Michigan University College of Medicine, Saginaw, MI; 2CHI Health Creighton School of Medicine, Omaha, NE; 3Rochester General Hospital, Rochester, NY; 4University of Utah School of Medicine, Salt Lake City, UT; 5Mayo Clinic, Rochester, MN; 6University of Nebraska Medical Center, Omaha, NE; 7Northwestern University, Chicago, IL; 8Allegheny Health Network, Pittsburgh, PA
Introduction: Global incidence and prevalence of inflammatory bowel disease (IBD) continues to rise, especially for individuals ≥60 years of age. There remains paucity of data on the use of biologics like Vedolizumab in elderly patients, as they are underrepresented in clinical trials. We performed a systematic review and meta-analysis to provide real-world data on the clinical efficacy and safety of Vedolizumab in the elderly population.
Methods: A systematic search of several databases including Ovid EBM reviews, Ovid Embase, Ovid Medline, Scopus, and Web of Science was performed through May 2022 to identify studies which assessed the safety and efficacy of Vedolizumab therapy in the elderly population. Pooled proportion and risk ratios (RR) were calculated using the random-effects model. Heterogeneity was assessed using Cochran Q statistical test and I2 statistics.
Results: A total of 11 studies with 3,546 IBD patients were included in our final analysis (1,314 elderly and 2,232 young). Mean age at initiation of vedolizumab therapy was 63.6–72.2 years for the elderly cohort.
Pooled rate of endoscopic, clinical, and steroid-free remission for elderly IBD patients was 32.69% (95% CI: 27.71-38.09; I2 93%), 37.95% (95% CI: 33.08-43.06; I2 13%) and 38.7% (95% CI: 31.63-46.43; I2 57%), respectively. Although elderly patients had lower rates of steroid-free remission, we did not find a statistically significant difference in rates of clinical or endoscopic remission when compared to younger patients.
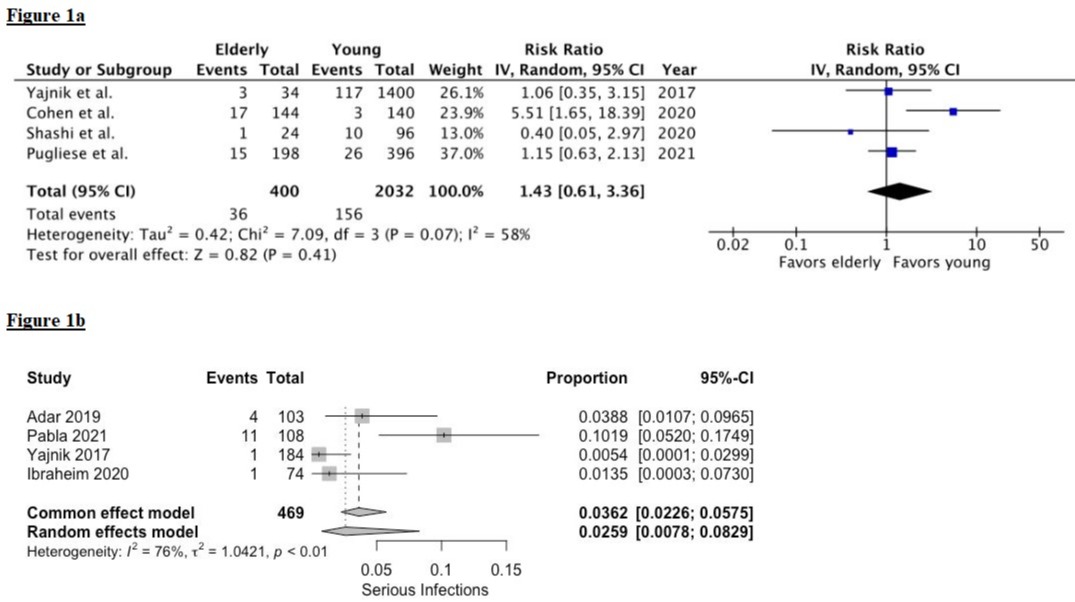
The pooled rate of overall and serious infections in the elderly cohort was 8.6% (95% CI: 6.83-10.8; I2 23%) and 3.6% (95% CI: 2.26-5.75; I2 76%), respectively. Pooled rate of IBD-related surgery and IBD-related hospitalizations was 8.64% (95% CI: 6.97-10.65; I2 78%) and 10.54% (95% CI: 8.37-13.2; I2 0%), respectively. There was no statistical difference in overall infections and IBD-related surgeries between elderly and young IBD patients, RR 1.43 (95% CI: 0.61-3.36; I2 58%), p=0.4 and RR 1.20 (95% CI: 0.79-1.84; I2 16%), p=0.4, respectively.
Discussion: Our analysis demonstrates that when compared to young IBD patients, Vedolizumab is equally safe and efficacious in inducing clinical and endoscopic remission in elderly patients.
Disclosures:
Dushyant S. Dahiya, MD1, Saurabh Chandan, MD2, Jay Bapaye, MD3, Babu P. Mohan, MD, MS4, Daryl Ramai, MD, MPH4, Lena L. Kassab, MD5, Ojasvini C. Chandan, MD6, Parambir S. Dulai, MD7, Gursimran S. Kochhar, MD8. C0390 - Safety and Efficacy of Vedolizumab in Elderly Patients With Inflammatory Bowel Disease: A Systematic Review and Meta-Analysis, ACG 2022 Annual Scientific Meeting Abstracts. Charlotte, NC: American College of Gastroenterology.
1Central Michigan University College of Medicine, Saginaw, MI; 2CHI Health Creighton School of Medicine, Omaha, NE; 3Rochester General Hospital, Rochester, NY; 4University of Utah School of Medicine, Salt Lake City, UT; 5Mayo Clinic, Rochester, MN; 6University of Nebraska Medical Center, Omaha, NE; 7Northwestern University, Chicago, IL; 8Allegheny Health Network, Pittsburgh, PA
Introduction: Global incidence and prevalence of inflammatory bowel disease (IBD) continues to rise, especially for individuals ≥60 years of age. There remains paucity of data on the use of biologics like Vedolizumab in elderly patients, as they are underrepresented in clinical trials. We performed a systematic review and meta-analysis to provide real-world data on the clinical efficacy and safety of Vedolizumab in the elderly population.
Methods: A systematic search of several databases including Ovid EBM reviews, Ovid Embase, Ovid Medline, Scopus, and Web of Science was performed through May 2022 to identify studies which assessed the safety and efficacy of Vedolizumab therapy in the elderly population. Pooled proportion and risk ratios (RR) were calculated using the random-effects model. Heterogeneity was assessed using Cochran Q statistical test and I2 statistics.
Results: A total of 11 studies with 3,546 IBD patients were included in our final analysis (1,314 elderly and 2,232 young). Mean age at initiation of vedolizumab therapy was 63.6–72.2 years for the elderly cohort.
Pooled rate of endoscopic, clinical, and steroid-free remission for elderly IBD patients was 32.69% (95% CI: 27.71-38.09; I2 93%), 37.95% (95% CI: 33.08-43.06; I2 13%) and 38.7% (95% CI: 31.63-46.43; I2 57%), respectively. Although elderly patients had lower rates of steroid-free remission, we did not find a statistically significant difference in rates of clinical or endoscopic remission when compared to younger patients.
The pooled rate of overall and serious infections in the elderly cohort was 8.6% (95% CI: 6.83-10.8; I2 23%) and 3.6% (95% CI: 2.26-5.75; I2 76%), respectively. Pooled rate of IBD-related surgery and IBD-related hospitalizations was 8.64% (95% CI: 6.97-10.65; I2 78%) and 10.54% (95% CI: 8.37-13.2; I2 0%), respectively. There was no statistical difference in overall infections and IBD-related surgeries between elderly and young IBD patients, RR 1.43 (95% CI: 0.61-3.36; I2 58%), p=0.4 and RR 1.20 (95% CI: 0.79-1.84; I2 16%), p=0.4, respectively.
Discussion: Our analysis demonstrates that when compared to young IBD patients, Vedolizumab is equally safe and efficacious in inducing clinical and endoscopic remission in elderly patients.

Figure: Figure 1a: Risk Ratio (RR) of overall infections. Figure 1b: Pooled rates of serious infections.
Disclosures:
Dushyant Dahiya indicated no relevant financial relationships.
Saurabh Chandan indicated no relevant financial relationships.
Jay Bapaye indicated no relevant financial relationships.
Babu Mohan indicated no relevant financial relationships.
Daryl Ramai indicated no relevant financial relationships.
Lena Kassab indicated no relevant financial relationships.
Ojasvini Chandan indicated no relevant financial relationships.
Parambir Dulai: BMS – Consultant. DigbiHealth – Stock Options. Gilead – Consultant. Janssen – Consultant. Lily – Consultant. Novartis – Consultant. Pfizer – Consultant, Grant/Research Support. PreciDiag – Royalties. Takeda – Consultant, Grant/Research Support.
Gursimran Kochhar: GIE Medical – Advisor or Review Panel Member. Lilly Pharma – Advisor or Review Panel Member.
Dushyant S. Dahiya, MD1, Saurabh Chandan, MD2, Jay Bapaye, MD3, Babu P. Mohan, MD, MS4, Daryl Ramai, MD, MPH4, Lena L. Kassab, MD5, Ojasvini C. Chandan, MD6, Parambir S. Dulai, MD7, Gursimran S. Kochhar, MD8. C0390 - Safety and Efficacy of Vedolizumab in Elderly Patients With Inflammatory Bowel Disease: A Systematic Review and Meta-Analysis, ACG 2022 Annual Scientific Meeting Abstracts. Charlotte, NC: American College of Gastroenterology.
