Poster Session B - Monday Morning
B0360 - Adverse Events and Serological Responses Following SARS-CoV-2 Vaccination in Individuals With Inflammatory Bowel Disease
- AM
Ante Markovinovic, BA
University of Calgary
Calgary, AB, Canada
Presenting Author(s)
1University of Calgary, Calgary, AB, Canada; 2University of Alberta, Edmonton, AB, Canada; 3University of Manitoba, Winnipeg, MB, Canada; 4McGill University, Montreal, PQ, Canada; 5University of Toronto, Toronto, ON, Canada
Introduction: The rapid development and distribution of SARS-CoV-2 vaccines has raised concerns surrounding vaccine safety in immunocompromised populations, such as those with inflammatory bowel disease (IBD). We described adverse events (AEs) following SARS-CoV-2 vaccination in those with IBD and to determine any relationship of AEs to post-vaccination antibody titres.
Methods: Individuals with IBD from a prospective cohort in Calgary, Canada who received a first, second, third, and/or fourth dose of a SARS-CoV-2 vaccine (Pfizer-BioNTech, Moderna, and/or AstraZeneca) were assessed for serological response and interviewed via telephone for AEs using questions based on the Adverse Events Following Immunization form. Subsequently, we used the Wilcoxon rank-sum test to analyze AEs and geometric mean titers (GMT). Interview and chart review were used to assess a flare of IBD within 30 days of vaccination.
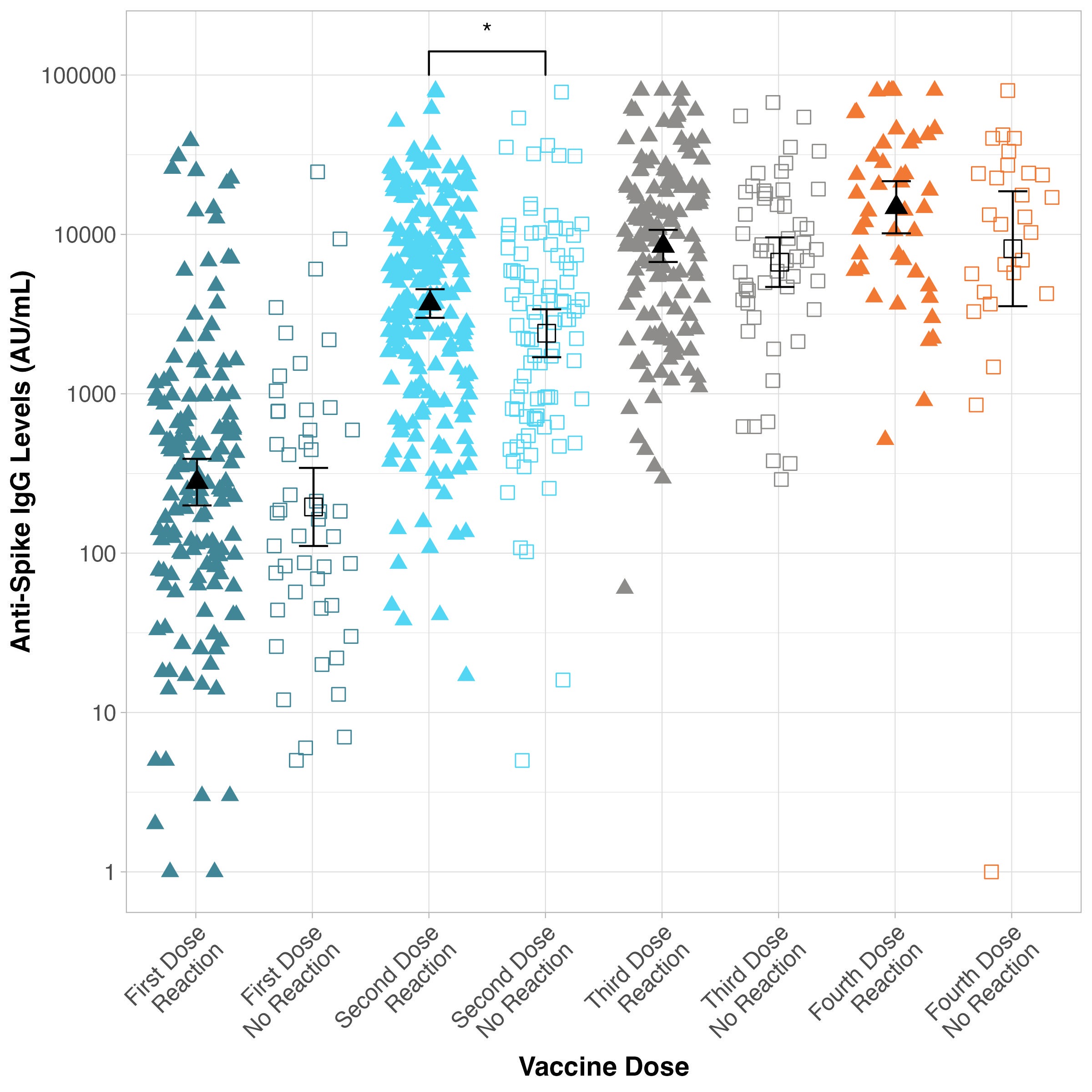
Results: Table 1 describes characteristics of individuals with IBD following the first dose (n=331), second dose (n=331), third dose (n=195), and fourth dose (n=100) of a SARS-CoV-2 vaccine. AEs were reported in 83.3% of participants after first dose, 79.1% following second dose, 77.4% following third dose, and 67.0% following fourth dose (Table 1). Injection site reaction (pain, redness, etc.) was the most common AE (50.8% of total AEs), with fatigue and malaise (18.1% of total AEs), headache and migraine (8.6% of total AEs), musculoskeletal discomfort (8.2% of total AEs), and fever and chills (6.5% of total AEs) also commonly reported. Only one participant was diagnosed with a severe AE requiring hospitalization: immune thrombocytopenic purpura (ITP) following their second dose of a Pfizer vaccine. No cases of IBD flare occurred within 30 days of a vaccine. Analysis found elevated GMT levels in those with injection site reactions compared to those without injection site reactions following all four doses, with second dose serological responses being statistically significantly different (8614/mL vs 6841 AU/mL [p< 0.05], respectively) (Figure 1).
Discussion: AEs following SARS-CoV-2 vaccination are generally mild and become less common with each consecutive dose. Antibody titres may be higher for participants who report injection site reactions compared to those without injections site reactions after second dose. Vaccines did not appear to be associated with a flare of IBD within 30 days of vaccination.
Characteristics | Dose 1 (/331) | Dose 2 (/331) | Dose 3 (/195) | Dose 4 (/100) |
Sex, n (%) Male Female |
155 (46.8%) 176 (53.2%) |
155 (46.8%) 176 (53.2%) |
86 (44.1%) 109 (55.9%) |
49 (49.0%) 51 (51.0%) |
Mean age (SD) | 52.05 (14.52) | 52.05 (14.52) | 51.81 (15.20) | 57.98 (14.01) |
IBD Type, n (%) Crohn’s Disease Ulcerative Colitis & IBD-U |
238 (71.9%) 93 (28.1%) |
238 (71.9%) 93 (28.1%) |
150 (76.9%) 45 (23.1%) |
75 (75.0%) 25 (25.0%) |
Medication, n (%) No immunosuppressives Anti-TNF only† Immunomodulators only Vedolizumab only Ustekinumab only Tofacitinib only Combination therapy‡ Oral Corticosteroids |
33 (10.0%) 118 (35.7%) 7 (2.1%) 37 (11.2%) 76 (23.0%) 5 (1.5%) 49 (14.8%) 6 (1.8%) |
32 (9.7%) 119 (36.0%) 7 (2.1%) 39 (11.8%) 74 (22.4%) 5 (1.5%) 47 (14.2%) 8 (2.4%) |
14 (7.2%) 74 (38.0%) 5 (2.6%) 19 (9.7%) 42 (21.5%) < 5 36 (18.5%) < 5 |
27 (27.0%) 20 (20.0%) < 5 9 (9.0%) 16 (16.0%) — 18 (18.0%) 7 (7.0%) |
Vaccine Type, n (%) Pfizer Moderna AstraZeneca |
271 (81.9%) 45 (13.6%) 15 (4.5%) |
275 (83.1%) 49 (14.8%) 7 (2.1%) |
179 (91.8%) 16 (8.2%) — |
82 (82.0%) 18 (18.0%) — |
Adverse Events | Dose 1 (/331) | Dose 2 (/331) | Dose 3 (/195) | Dose 4 (/100) |
Injection site, n (%) | 250 (75.5%) | 231 (70%) | 138 (70.8%) | 55 (55.0%) |
Lymph node swelling, n (%) | 1 (0.3%) | 9 (2.7%) | 14 (7.2%) | 1 (1.0%) |
Gastrointestinal, n (%) | 17 (5.1%) | 16 (4.8%) | 6 (3.1%) | 2 (2.0%) |
Fatigue or malaise, n (%) | 86 (26.0%) | 87 (26.3%) | 45 (23.1%) | 22 (22.0%) |
Fever or chills, n (%) | 27 (8.2%) | 35 (10.6%) | 19 (9.7%) | 5 (5.0%) |
Musculoskeletal, n (%) | 34 (10.3%) | 41 (12.4%) | 25 (12.8%) | 9 (9.0%) |
Headache or migraine, n (%) | 34 (10.3%) | 46 (13.9%) | 23 (11.8%) | 11 (11.0%) |
Other, n (%) | 16 (4.8%)α | 14 (4.2%)β | 7 (3.6%)γ | 2 (2.0%)δ |
Any symptoms, n (%) | 275 (83.3%) | 261 (79.1%) | 151 (77.4%) | 67 (67.0%) |
†One of golimumab, adalimumab, or infliximab (originator or biosimilar)
‡Any combination of anti-TNF and one or more of the following therapies: vedolizumab, ustekinumab, tofacitinib, azathioprine, 6-mercaptopurine, or methotrexate
αParesthesia, chin swelling, dysgeusia, numbness, hot flashes, irritability, ennui, hyperactivity, brain fog, congestion, dry eyes, sleep trouble, testicular swelling, angioedema, sinus swelling, throat swelling
βShingles, hot flashes, brain fog, sleep troubles, rapid heartbeat, forced breathing, congestion, angioedema, throat swelling, leg swelling
γHot flashes, brain fog, sleep troubles, sore throat, congestion, angioedema, ITP
δParesthesia, sleep troubles
Disclosures:
Ante Markovinovic, BA1, Michelle Herauf, BSc1, Joshua Quan, BKin1, Lindsay Hracs, PhD1, Joseph Windsor, PhD1, Nastaran Sharifi, MD1, Stephanie Coward, PhD1, Léa Caplan, BHSc1, Christopher Ma, MD1, Remo Panaccione, MD1, Richard Ingram, MD, PhD1, Jamil Kanji, MD1, Graham Tipples, PhD2, Jessalyn Holodinsky, PhD1, Charles Bernstein, MD3, Douglas Mahoney, PhD1, Sasha Bernatsky, MD4, Eric Benchimol, MD, PhD5, Gilaad Kaplan, MD, MPH1. B0360 - Adverse Events and Serological Responses Following SARS-CoV-2 Vaccination in Individuals With Inflammatory Bowel Disease, ACG 2022 Annual Scientific Meeting Abstracts. Charlotte, NC: American College of Gastroenterology.
