Back
Poster Session E - Tuesday Afternoon
E0201 - The Efficacy and Safety of PD-1/PD-L1 Inhibitors for Advanced Refractory Esophageal and GE Junction Cancer: A Meta-Analysis
Tuesday, October 25, 2022
3:00 PM – 5:00 PM ET
Location: Crown Ballroom

Tian Li, MD, MS
SUNY Downstate Health Sciences University
Brooklyn, NY
Presenting Author(s)
Tian Li, MD, MS1, Yuntao Zou, MD2, Yiting Li, MD3, Frank Friedenberg, MD, MS4
1SUNY Downstate Health Sciences University, Brooklyn, NY; 2Saint Peter's University Hospital, New Brunswick, NJ; 3University of New Mexico Health Sciences Center, Albuquerque, NM; 4Lewis Katz School of Medicine at Temple University, Philadelphia, PA
Introduction: Anti-programmed death-1 (PD-1) and programmed death-ligand (PD-L1) immunotherapy have been studied as adjuvant and neoadjuvant treatment for patients with advanced esophageal or gastro-esophageal junction (GEJ) cancer. Our aim was to use the highest quality data available to perform a meta-analysis evaluating their efficacy and safety.
Methods: We reviewed PubMed, MEDLINE, Embase, and Web of Science Core Collection databases from inception to Aug 1st, 2021, to identify studies evaluating the efficacy and safety profile of PD-1 and PD-L1 inhibitor immunotherapy in the management of advanced refractory esophageal or GEJ cancer. Our primary outcome was overall survival. Secondary outcomes were progression-free survival, and objective response including complete response, partial response, stable disease, and progressive disease. Adverse effects were characterized according to Common Terminology Criteria for Adverse Events v 4.0. Pooled rates with 95% confidence intervals (CI) for all outcomes were calculated using a random-effect model.
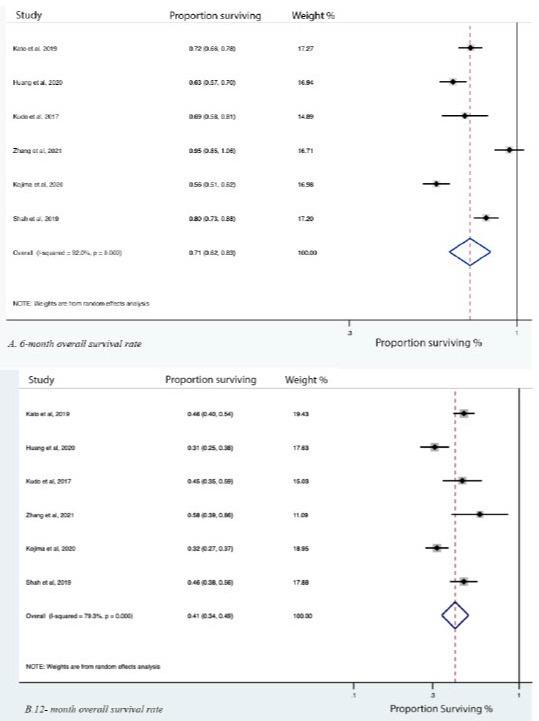
Results: Literature review identified 12 randomized clinical trial articles and one retrospective chart review study suitable for meta-analysis. Most studies were performed in East Asia. The median age of participants from all studies ranged from 60 to 65 years The majority of patients included in the studies had stage III or stage IV disease. Pooled 6- and 12- month overall survival rates were 71% (95% CI 62%-83%) and 41% (95% CI 34%-49%), respectively (Figure 1). Pooled 6- and 12- month progression-free survival rates were 30% (18%-52%) and 15% (7%-35%), respectively (Figure 1). Response rates were as follows: complete response 4% (1%-14%); partial response 30% (17%-52%); stable disease 23% (19%-27%,) and progressive disease 48% (39%-59%). The most common side effect was constipation. It occurred in 17% (7%-41%) and was graded 1-2 (mild) in all cases. Other common adverse effects were diarrhea, anorexia, nausea, and vomiting (Figure 2). The most common non-GI adverse effect was fatigue, with an overall rate of 16% (9%-28%). Fatigue was graded as 1-2 in 94% of cases. The other common non-GI side effects included hypothyroidism and rash.
Discussion: In summary, use of PD-1/PD-L1 inhibitors provides clinically meaningful rates of survival and response. They demonstrate a well-tolerated safety profile thus supporting their current role as adjuvant or neoadjuvant therapy for advanced esophageal and GEJ cancer.
Disclosures:
Tian Li, MD, MS1, Yuntao Zou, MD2, Yiting Li, MD3, Frank Friedenberg, MD, MS4. E0201 - The Efficacy and Safety of PD-1/PD-L1 Inhibitors for Advanced Refractory Esophageal and GE Junction Cancer: A Meta-Analysis, ACG 2022 Annual Scientific Meeting Abstracts. Charlotte, NC: American College of Gastroenterology.
1SUNY Downstate Health Sciences University, Brooklyn, NY; 2Saint Peter's University Hospital, New Brunswick, NJ; 3University of New Mexico Health Sciences Center, Albuquerque, NM; 4Lewis Katz School of Medicine at Temple University, Philadelphia, PA
Introduction: Anti-programmed death-1 (PD-1) and programmed death-ligand (PD-L1) immunotherapy have been studied as adjuvant and neoadjuvant treatment for patients with advanced esophageal or gastro-esophageal junction (GEJ) cancer. Our aim was to use the highest quality data available to perform a meta-analysis evaluating their efficacy and safety.
Methods: We reviewed PubMed, MEDLINE, Embase, and Web of Science Core Collection databases from inception to Aug 1st, 2021, to identify studies evaluating the efficacy and safety profile of PD-1 and PD-L1 inhibitor immunotherapy in the management of advanced refractory esophageal or GEJ cancer. Our primary outcome was overall survival. Secondary outcomes were progression-free survival, and objective response including complete response, partial response, stable disease, and progressive disease. Adverse effects were characterized according to Common Terminology Criteria for Adverse Events v 4.0. Pooled rates with 95% confidence intervals (CI) for all outcomes were calculated using a random-effect model.
Results: Literature review identified 12 randomized clinical trial articles and one retrospective chart review study suitable for meta-analysis. Most studies were performed in East Asia. The median age of participants from all studies ranged from 60 to 65 years The majority of patients included in the studies had stage III or stage IV disease. Pooled 6- and 12- month overall survival rates were 71% (95% CI 62%-83%) and 41% (95% CI 34%-49%), respectively (Figure 1). Pooled 6- and 12- month progression-free survival rates were 30% (18%-52%) and 15% (7%-35%), respectively (Figure 1). Response rates were as follows: complete response 4% (1%-14%); partial response 30% (17%-52%); stable disease 23% (19%-27%,) and progressive disease 48% (39%-59%). The most common side effect was constipation. It occurred in 17% (7%-41%) and was graded 1-2 (mild) in all cases. Other common adverse effects were diarrhea, anorexia, nausea, and vomiting (Figure 2). The most common non-GI adverse effect was fatigue, with an overall rate of 16% (9%-28%). Fatigue was graded as 1-2 in 94% of cases. The other common non-GI side effects included hypothyroidism and rash.
Discussion: In summary, use of PD-1/PD-L1 inhibitors provides clinically meaningful rates of survival and response. They demonstrate a well-tolerated safety profile thus supporting their current role as adjuvant or neoadjuvant therapy for advanced esophageal and GEJ cancer.

Figure: Table 1. Overall survival rate at 6- and 12- month in patients taking PD-1/PD-L1 inhibitors
Disclosures:
Tian Li indicated no relevant financial relationships.
Yuntao Zou indicated no relevant financial relationships.
Yiting Li indicated no relevant financial relationships.
Frank Friedenberg indicated no relevant financial relationships.
Tian Li, MD, MS1, Yuntao Zou, MD2, Yiting Li, MD3, Frank Friedenberg, MD, MS4. E0201 - The Efficacy and Safety of PD-1/PD-L1 Inhibitors for Advanced Refractory Esophageal and GE Junction Cancer: A Meta-Analysis, ACG 2022 Annual Scientific Meeting Abstracts. Charlotte, NC: American College of Gastroenterology.
