Back
Poster Session E - Tuesday Afternoon
E0151 - Gastric Ulcerations With the Newer Pill-Based Bowel Preparations for Colonoscopy: Modifications to Improve Patient Outcomes
Tuesday, October 25, 2022
3:00 PM – 5:00 PM ET
Location: Crown Ballroom
- NV
Neal Vasireddi, BA
Cornell University
Metuchen, NJ
Presenting Author(s)
Henrik A. Hahamyan, BS1, Akhil Chennuru, 2, Neal S. Vasireddi, BA3, Navya Sharma, 4, Shray Awasti, 5, Kavya Sudireddy, 6, Srinivas S. Vasireddi, MD7, Nikhil Vasireddi, MHA8
1Vanderbilt University Medical Center, Nashville, TN; 2University of the Sciences in Philadelphia, Cranbury, NJ; 3Cornell University, Metuchen, NJ; 4University of California, Santa Barbara, Santa Barbara, CA; 5New York University, Cranbury, NJ; 6Apollo Institute of Medical Sciences and Research, Hyderabad, Andhra Pradesh, India; 7Advanced Digestive Center, Inc., Metuchen, NJ; 8Case Western Reserve University School of Medicine, Metuchen, NJ
Introduction: Pill-based preparations for endoscopy have become increasingly popular relative to liquid preparations due to patient preference and cleansing efficacy. SUTAB® (sodium sulfate, magnesium sulfate, and potassium chloride Tablets) is a newly FDA approved pill-based endoscopy preparation with a 92% successful cleansing rate in trials. SUTAB® on-label administration recommends consuming 12 tablets with 16 ounces of water within 30 minutes, followed by 32 ounces of water on the day before and morning prior to pan-endoscopy. However, rapid consumption of tablets has been associated with chemically-induced ulceration. Here, we discuss two cases who underwent pan-endoscopy for iron deficiency anemia with findings of acute ulcerations and suggest recommendations for staggered pill consumption to improve patient outcomes.
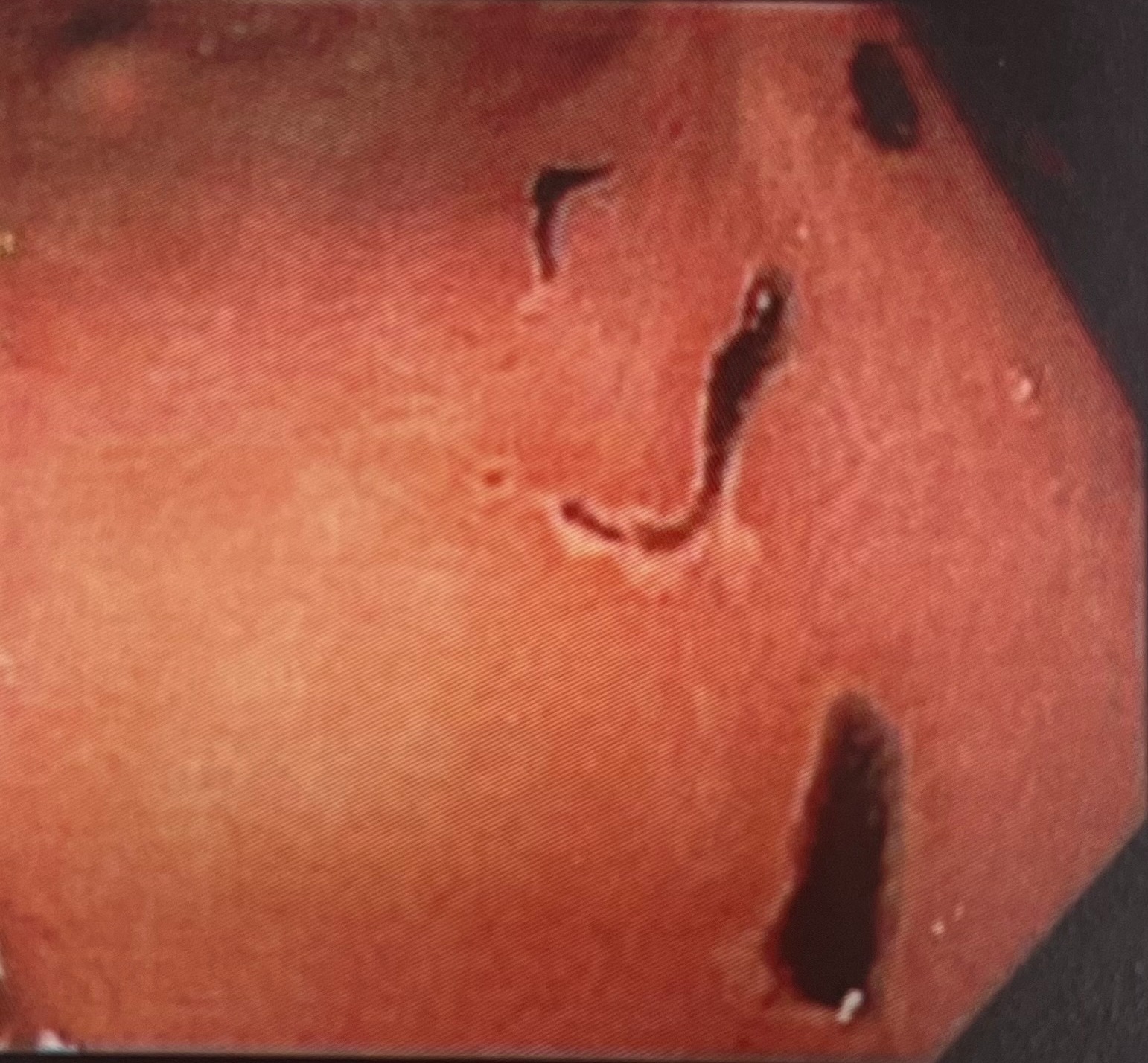
Case Description/Methods: In case #1 (male, age 46), pan-endoscopy was indicated for anemia, abdominal distention and mild gastroesophageal reflux. Relevant findings on upper endoscopy were moderate gastritis and antral erosions/ulcerations (image 1). In case #2 (female, age 54), a pan-endoscopy was indicated for iron deficiency anemia. Relevant findings on upper exam included moderate gastritis, multiple antral erosions, and healing superficial antral ulcers with eschar. Standard SUTAB® on-label administration was followed. In the absence of prior symptoms/other causative etiology, erosions and ulcerations were deemed drug-induced. In all subsequent cases at our center warranting pan-endoscopy , we modified the manufacturer specified SUTAB® administration protocol, which involved consuming each of the 12 pills 5 minutes apart with 25 ounces of water, both on the day before and morning prior to pan-endoscopy. This staggered modified administration led to complete alleviation of the noted erosions/ulcerations in all subsequent cases.
Discussion: Although pill-based endoscopy preparations are convenient, the current on-label stacked administration recommendations may cause gastric ulcerations. Physicians using the revised on-label SUTAB® administration may still find unexpected ulcerations/erosions without other causative etiology. An off-label modification of administration to further stagger the intake of each pill over 5 minutes (as opposed to the package recommended 2.5 minutes) may improve noted endoscopic patient outcomes and should be considered. The optimal pill intake protocols however need to be defined in studies in larger pool of patients.
Disclosures:
Henrik A. Hahamyan, BS1, Akhil Chennuru, 2, Neal S. Vasireddi, BA3, Navya Sharma, 4, Shray Awasti, 5, Kavya Sudireddy, 6, Srinivas S. Vasireddi, MD7, Nikhil Vasireddi, MHA8. E0151 - Gastric Ulcerations With the Newer Pill-Based Bowel Preparations for Colonoscopy: Modifications to Improve Patient Outcomes, ACG 2022 Annual Scientific Meeting Abstracts. Charlotte, NC: American College of Gastroenterology.
1Vanderbilt University Medical Center, Nashville, TN; 2University of the Sciences in Philadelphia, Cranbury, NJ; 3Cornell University, Metuchen, NJ; 4University of California, Santa Barbara, Santa Barbara, CA; 5New York University, Cranbury, NJ; 6Apollo Institute of Medical Sciences and Research, Hyderabad, Andhra Pradesh, India; 7Advanced Digestive Center, Inc., Metuchen, NJ; 8Case Western Reserve University School of Medicine, Metuchen, NJ
Introduction: Pill-based preparations for endoscopy have become increasingly popular relative to liquid preparations due to patient preference and cleansing efficacy. SUTAB® (sodium sulfate, magnesium sulfate, and potassium chloride Tablets) is a newly FDA approved pill-based endoscopy preparation with a 92% successful cleansing rate in trials. SUTAB® on-label administration recommends consuming 12 tablets with 16 ounces of water within 30 minutes, followed by 32 ounces of water on the day before and morning prior to pan-endoscopy. However, rapid consumption of tablets has been associated with chemically-induced ulceration. Here, we discuss two cases who underwent pan-endoscopy for iron deficiency anemia with findings of acute ulcerations and suggest recommendations for staggered pill consumption to improve patient outcomes.
Case Description/Methods: In case #1 (male, age 46), pan-endoscopy was indicated for anemia, abdominal distention and mild gastroesophageal reflux. Relevant findings on upper endoscopy were moderate gastritis and antral erosions/ulcerations (image 1). In case #2 (female, age 54), a pan-endoscopy was indicated for iron deficiency anemia. Relevant findings on upper exam included moderate gastritis, multiple antral erosions, and healing superficial antral ulcers with eschar. Standard SUTAB® on-label administration was followed. In the absence of prior symptoms/other causative etiology, erosions and ulcerations were deemed drug-induced. In all subsequent cases at our center warranting pan-endoscopy , we modified the manufacturer specified SUTAB® administration protocol, which involved consuming each of the 12 pills 5 minutes apart with 25 ounces of water, both on the day before and morning prior to pan-endoscopy. This staggered modified administration led to complete alleviation of the noted erosions/ulcerations in all subsequent cases.
Discussion: Although pill-based endoscopy preparations are convenient, the current on-label stacked administration recommendations may cause gastric ulcerations. Physicians using the revised on-label SUTAB® administration may still find unexpected ulcerations/erosions without other causative etiology. An off-label modification of administration to further stagger the intake of each pill over 5 minutes (as opposed to the package recommended 2.5 minutes) may improve noted endoscopic patient outcomes and should be considered. The optimal pill intake protocols however need to be defined in studies in larger pool of patients.

Figure: image1: acute antral ulcerations
Disclosures:
Henrik Hahamyan indicated no relevant financial relationships.
Akhil Chennuru indicated no relevant financial relationships.
Neal Vasireddi indicated no relevant financial relationships.
Navya Sharma indicated no relevant financial relationships.
Shray Awasti indicated no relevant financial relationships.
Kavya Sudireddy indicated no relevant financial relationships.
Srinivas Vasireddi indicated no relevant financial relationships.
Nikhil Vasireddi indicated no relevant financial relationships.
Henrik A. Hahamyan, BS1, Akhil Chennuru, 2, Neal S. Vasireddi, BA3, Navya Sharma, 4, Shray Awasti, 5, Kavya Sudireddy, 6, Srinivas S. Vasireddi, MD7, Nikhil Vasireddi, MHA8. E0151 - Gastric Ulcerations With the Newer Pill-Based Bowel Preparations for Colonoscopy: Modifications to Improve Patient Outcomes, ACG 2022 Annual Scientific Meeting Abstracts. Charlotte, NC: American College of Gastroenterology.

