Back

Poster Session A - Sunday Afternoon
Category: IBD
A0394 - Efficacy and Safety of Ustekinumab for Ulcerative Colitis Through 4 Years: Final Results From the UNIFI Long-Term Extension
Sunday, October 23, 2022
5:00 PM – 7:00 PM ET
Location: Crown Ballroom

Has Audio

Bruce E. Sands, MD, MS, FACG
Icahn School of Medicine at Mount Sinai
New York, NY
Presenting Author(s)
Bruce E. Sands, MD, MS, FACG1, Maria T. Abreu, MD2, Silvio Danese, MD, PhD3, William J. Sandborn, MD4, Ye Miao, MS5, Hongyan Zhang, PhD5, Remo Panaccione, MD6, Tadakazu Hisamatsu, MD, PhD7, Ellen J. Scherl, MD8, Rupert W. Leong, MD9, David S. Rowbotham, MD10, Ramesh P. Arasaradnam, PhD11, Laurent Peyrin-Biroulet, MD, PhD12, Waqqas Afif, MD13, Colleen Marano, PhD5
1Icahn School of Medicine at Mount Sinai, New York, NY; 2The University of Miami Health System, Miami, FL; 3IRCCS Ospedale San Raffaele and University Vita-Salute San Raffaele, Milan, Molise, Italy; 4University of California San Diego, La Jolla, CA; 5Janssen Research & Development, LLC, Spring House, PA; 6University of Calgary, Calgary, AB, Canada; 7Kyorin University School of Medicine, Tokyo, Tokyo, Japan; 8Weill Cornell Medicine, New York Presbyterian Hospital, New York, NY; 9Concord Hospital and Macquarie University Hospital, Sydney, New South Wales, Australia; 10Auckland City Hospital, Auckland, Auckland, New Zealand; 11Warwick Medical School, University of Warwick & University Hospital Coventry, Coventry Warwickshire, England, United Kingdom; 12University of Lorraine, Nancy, Lorraine, France; 13McGill University Health Centre, Montreal, PQ, Canada
Introduction: Ustekinumab (UST) is approved for moderate to severe ulcerative colitis (UC) treatment. Here we report final results of the UNIFI long-term extension (LTE) study with efficacy and safety through 4 years (yrs) of subcutaneous (SC) UST treatment.
Methods: Overall, 523 intravenous UST induction responders were randomized to SC maintenance therapy: 175 SC placebo (PBO); 172 UST 90mg every 12 weeks (q12w); 176 UST 90mg q8w. The nonrandomized population included UST induction nonresponders at week (wk) 8 who received SC UST, responded 8wks later and continued receiving UST q8w, and PBO induction responders continuing PBO. Patients (pts) completing wk44 were eligible to continue treatment in LTE. PBO pts discontinued after study unblinding. Starting at wk56, randomized pts with UC worsening could adjust to q8w. Efficacy was evaluated in UST-randomized pts (n=284) using symptomatic remission (Mayo stool frequency subscore 0/1 and rectal bleeding subscore 0). Safety was evaluated for all 588 pts treated in LTE (randomized/nonrandomized populations).
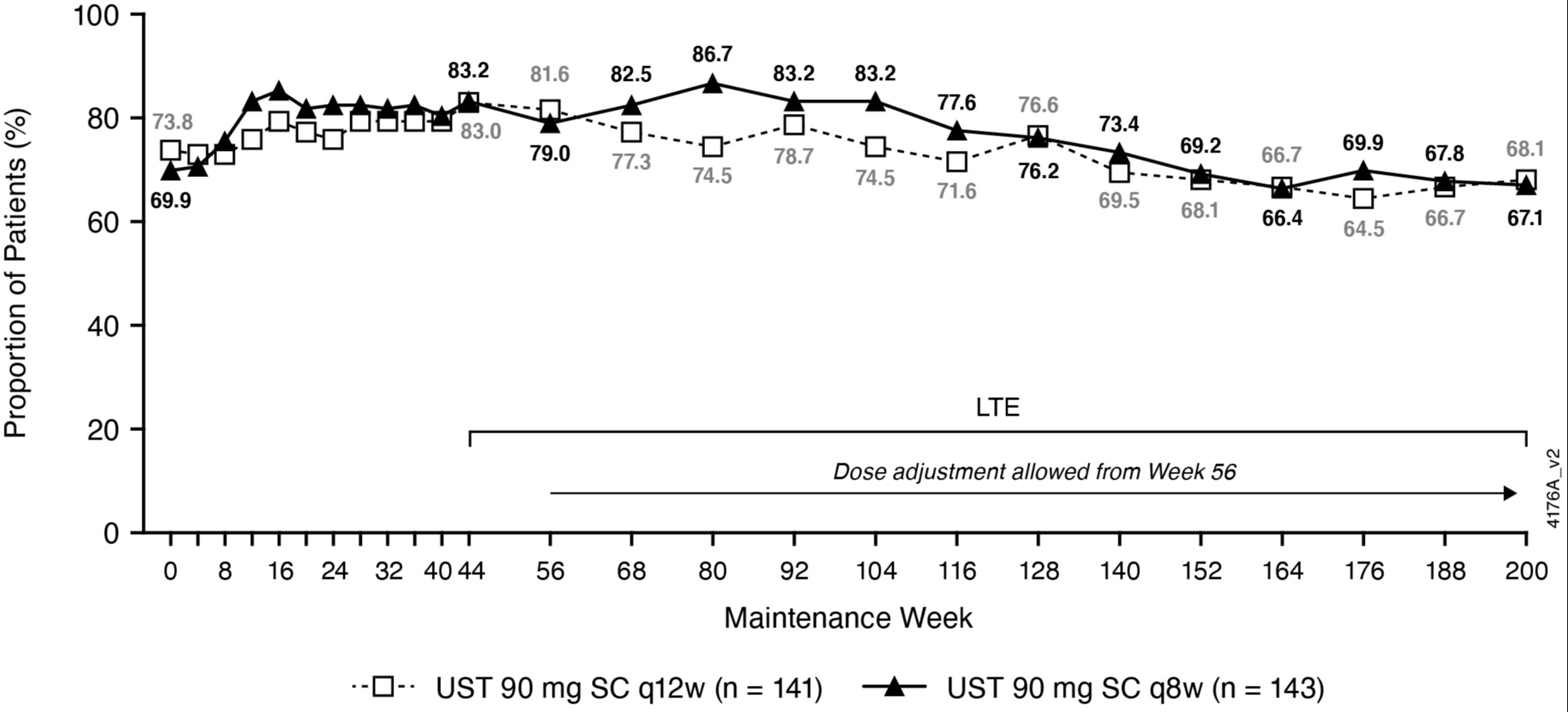
Results: Among all pts randomized to UST at maintenance baseline (intent-to-treat population with nonresponder imputation for missing data and treatment failure criteria), 55.2% were in symptomatic remission at wk200 (biologic naïve 67.2%; biologic failure 41.6%; Table 1); 53.2% achieved corticosteroid-free symptomatic remission at wk200. Overall, 42.7% of biologic failure and 18.8% of biologic naïve pts randomized to UST and treated in the LTE discontinued treatment between wks44 and 200. Among randomized pts who continued UST in the LTE, 67.6% were in symptomatic remission at wk200; 72.9% of those in clinical remission at wk44 were in symptomatic remission at wk200; and 85.1% of pts with observed data at wk200 were in symptomatic remission.
Safety events were similar between UST-treated pts and PBO throughout study. Maintenance wks0-220 included 1647.4 (UST) and 301.7 (PBO) pt yrs of follow-up. Safety events per 100 pt yrs of follow-up for UST vs PBO were adverse events (AEs): 214.45 vs 288.04, serious AEs: 7.22 vs 10.61, and serious infections: 2.00 vs 3.31. During the final yr of the LTE, no deaths or major cardiovascular events were reported in UST pts. Among UST pts, 2 cases of colorectal cancer and 1 case of cytomegalovirus were reported.
Discussion: Pts receiving SC UST generally maintained clinical benefit through 4 yrs. No new safety signals were observed.
Disclosures:
Bruce E. Sands, MD, MS, FACG1, Maria T. Abreu, MD2, Silvio Danese, MD, PhD3, William J. Sandborn, MD4, Ye Miao, MS5, Hongyan Zhang, PhD5, Remo Panaccione, MD6, Tadakazu Hisamatsu, MD, PhD7, Ellen J. Scherl, MD8, Rupert W. Leong, MD9, David S. Rowbotham, MD10, Ramesh P. Arasaradnam, PhD11, Laurent Peyrin-Biroulet, MD, PhD12, Waqqas Afif, MD13, Colleen Marano, PhD5. A0394 - Efficacy and Safety of Ustekinumab for Ulcerative Colitis Through 4 Years: Final Results From the UNIFI Long-Term Extension, ACG 2022 Annual Scientific Meeting Abstracts. Charlotte, NC: American College of Gastroenterology.
1Icahn School of Medicine at Mount Sinai, New York, NY; 2The University of Miami Health System, Miami, FL; 3IRCCS Ospedale San Raffaele and University Vita-Salute San Raffaele, Milan, Molise, Italy; 4University of California San Diego, La Jolla, CA; 5Janssen Research & Development, LLC, Spring House, PA; 6University of Calgary, Calgary, AB, Canada; 7Kyorin University School of Medicine, Tokyo, Tokyo, Japan; 8Weill Cornell Medicine, New York Presbyterian Hospital, New York, NY; 9Concord Hospital and Macquarie University Hospital, Sydney, New South Wales, Australia; 10Auckland City Hospital, Auckland, Auckland, New Zealand; 11Warwick Medical School, University of Warwick & University Hospital Coventry, Coventry Warwickshire, England, United Kingdom; 12University of Lorraine, Nancy, Lorraine, France; 13McGill University Health Centre, Montreal, PQ, Canada
Introduction: Ustekinumab (UST) is approved for moderate to severe ulcerative colitis (UC) treatment. Here we report final results of the UNIFI long-term extension (LTE) study with efficacy and safety through 4 years (yrs) of subcutaneous (SC) UST treatment.
Methods: Overall, 523 intravenous UST induction responders were randomized to SC maintenance therapy: 175 SC placebo (PBO); 172 UST 90mg every 12 weeks (q12w); 176 UST 90mg q8w. The nonrandomized population included UST induction nonresponders at week (wk) 8 who received SC UST, responded 8wks later and continued receiving UST q8w, and PBO induction responders continuing PBO. Patients (pts) completing wk44 were eligible to continue treatment in LTE. PBO pts discontinued after study unblinding. Starting at wk56, randomized pts with UC worsening could adjust to q8w. Efficacy was evaluated in UST-randomized pts (n=284) using symptomatic remission (Mayo stool frequency subscore 0/1 and rectal bleeding subscore 0). Safety was evaluated for all 588 pts treated in LTE (randomized/nonrandomized populations).
Results: Among all pts randomized to UST at maintenance baseline (intent-to-treat population with nonresponder imputation for missing data and treatment failure criteria), 55.2% were in symptomatic remission at wk200 (biologic naïve 67.2%; biologic failure 41.6%; Table 1); 53.2% achieved corticosteroid-free symptomatic remission at wk200. Overall, 42.7% of biologic failure and 18.8% of biologic naïve pts randomized to UST and treated in the LTE discontinued treatment between wks44 and 200. Among randomized pts who continued UST in the LTE, 67.6% were in symptomatic remission at wk200; 72.9% of those in clinical remission at wk44 were in symptomatic remission at wk200; and 85.1% of pts with observed data at wk200 were in symptomatic remission.
Safety events were similar between UST-treated pts and PBO throughout study. Maintenance wks0-220 included 1647.4 (UST) and 301.7 (PBO) pt yrs of follow-up. Safety events per 100 pt yrs of follow-up for UST vs PBO were adverse events (AEs): 214.45 vs 288.04, serious AEs: 7.22 vs 10.61, and serious infections: 2.00 vs 3.31. During the final yr of the LTE, no deaths or major cardiovascular events were reported in UST pts. Among UST pts, 2 cases of colorectal cancer and 1 case of cytomegalovirus were reported.
Discussion: Pts receiving SC UST generally maintained clinical benefit through 4 yrs. No new safety signals were observed.

Figure: Symptomatic Remission of Randomized Patients in Maintenance Who Were Treated in Long-Term Extension (LTE) Through Week 200 (a,b,c).
Symptomatic remission: Mayo stool frequency subscore of 0 or 1 and a rectal bleeding subscore of 0; SC: subcutaneous; q8w: every 8 weeks; q12w: every 12 weeks.
a) Patients who had both stool frequency and rectal bleeding subscores missing at a visit were considered not to be in symptomatic remission for that visit.
b) Randomized group at maintenance Week 0 regardless of whether patients had a dose adjustment during the long-term extension.
c) Patients who had an ostomy or colectomy, or discontinued study agent due to lack of therapeutic effect or due to an adverse event of worsening of ulcerative colitis prior to the designated visit were considered not to be in symptomatic remission.
Symptomatic remission: Mayo stool frequency subscore of 0 or 1 and a rectal bleeding subscore of 0; SC: subcutaneous; q8w: every 8 weeks; q12w: every 12 weeks.
a) Patients who had both stool frequency and rectal bleeding subscores missing at a visit were considered not to be in symptomatic remission for that visit.
b) Randomized group at maintenance Week 0 regardless of whether patients had a dose adjustment during the long-term extension.
c) Patients who had an ostomy or colectomy, or discontinued study agent due to lack of therapeutic effect or due to an adverse event of worsening of ulcerative colitis prior to the designated visit were considered not to be in symptomatic remission.
| Table 1. Symptomatic remissiona rates during the LTE in randomized patients | |||
| Analysis | 90 mg UST SC q12wb | 90 mg UST SC q8wb | Overall UST |
| Symptomatic remission in the ITT populationc,d,e (treatment failure and missing data nonresponder imputation) | |||
| Week 44, n/N (%) | 107/172 (62.2) | 119/176 (67.6) | 226/348 (64.9) |
| Week 200, n/N (%) | 96/172 (55.8) | 96/176 (54.5) | 192/348 (55.2) |
| Symptomatic remission in biologic naïvef patients | |||
| Week 44, n/N (%) | 68/95 (71.6) | 57/79 (72.2) | 125/174 (71.8) |
| Week 200, n/N (%) | 62/95 (65.3) | 55/79 (69.6) | 117/174 (67.2) |
| Symptomatic remission in biologic failuref patients | |||
| Week 44, n/N (%) | 34/70 (48.6) | 57/91 (62.6) | 91/161 (56.5) |
| Week 200, n/N (%) | 30/70 (42.9) | 37/91 (40.7) | 67/161 (41.6) |
| Corticosteroid-free symptomatic remission in the ITT populationc,d,e,g | |||
| Week 44, n/N (%) | 105/172 (61.0) | 116/176 (65.9) | 221/348 (63.5) |
| Week 200, n/N (%) | 94/172 (54.7) | 91/176 (51.7) | 185/348 (53.2) |
| Symptomatic remission in patients treated in the LTE (treatment failure and missing data nonresponder imputation)d,e | |||
| Week 44, n/N (%) | 117/141 (83.0) | 119/143 (83.2) | 236/284 (83.1) |
| Week 200, n/N (%) | 96/141 (68.1) | 96/143 (67.1) | 192/284 (67.6) |
| Symptomatic remission up to the time of dose adjustment with treatment failure rules applied in patients treated in the LTEd (modified as observedh) | |||
| Week 44, n/N (%) | 117/141 (83.0) | 119/143 (83.2) | 236/284 (83.1) |
| Week 200, n/N (%) | 58/65 (89.2) | 73/89 (82.0) | 131/154 (85.1) |
| AE, adverse event; ITT, intent-to-treat; LTE, long-term extension; q8w, every 8 weeks; q12w, every 12 weeks; SC, subcutaneous; TNF, tumor necrosis factor; UC, ulcerative colitis; UST, ustekinumab a Symptomatic remission is defined as a stool frequency subscore of 0 or 1 and a rectal bleeding subscore of 0. b Randomized group at maintenance Week 0 regardless of whether or not patients had a dose adjustment during the long-term extension. c Patients who had a prohibited change in UC medication, an ostomy or colectomy, or used a rescue medication after clinical flare, or discontinued study agent due to lack of therapeutic effect or due to an AE of worsening of UC prior to the Week 44 visit were considered not to be in symptomatic remission at Week 44. d Patients who had an ostomy or colectomy, or discontinued study agent due to lack of therapeutic effect or due to an AE of worsening of UC after Week 44 and prior to Week 200, were considered not to be in symptomatic remission at Week 200. e Patients who had both stool frequency and rectal bleeding subscores missing at a visit were considered not to be in symptomatic remission for that visit. f Efficacy was evaluated only in patients with a history of biologic failure to ≥1 biologic (anti-TNFα or integrin antagonist) and those who were biologic naïve; 7 UST q12w and 6 UST q8w patients who were biologic-experienced but did not have documentation of a history of biologic failure are excluded. g Patients who had a missing value in corticosteroid use had their last value carried forward. h The observed data excluded patients with missing data and had not had treatment failure (i.e., an ostomy or colectomy, or discontinued study agent due to lack of therapeutic effect or due to an AE of worsening of UC) prior to the designated visit. | |||
Disclosures:
Bruce Sands: Abivax – Consultant, Speaking. Amgen – Consultant. Arena Pharmaceuticals – Consultant. Artugen Therapeutics – Consultant. AstraZeneca – Consultant. Bacainn Therapeutics – Consultant. Boehringer-Ingelheim – Consultant. Boston Pharmaceuticals – Consultant. Bristol Myers Squibb – Consultant, speaking, research funding. Calibr – Consultant. Celltrion Healthcare – Consultant. ClostraBio – Consultant. Eli Lilly and Company – Consultant. Entera – Consultant. Evommune – Consultant. Galapagos – Consultant. Genentech – Consultant. Gilead Sciences – Consultant. GlaxoSmithKline – Consultant. Gossamer Bio – Consultant. InDex Pharmaceuticals – Consultant. Innovation Therapeutics – Consultant. Inotrem – Consultant. Ironwood Pharmaceuticals – Consultant. Janssen – Consultant, Grant/Research Support, speaking. Kaleido – Consultant. Kallyope – Consultant. Miro Bio – Consultant. Morphic Therapeutics – Consultant. MRM Health – Consultant. Pfizer – Consultant, speaking. Progenity – Consultant. Prometheus Biosciences – Consultant. Protagonist Therapeutics – Consultant. Q32 Bio – Consultant. Surrozen – Consultant. Takeda – Consultant, Speaking. Teva – Consultant. TLL Pharmaceutical – Consultant. USWM Enterprises – Consultant. VielaBio – Consultant.
Maria Abreu: AbbVie – Advisory Committee/Board Member, Consultant. Alimentiv – Speakers Bureau. Arena Pharmaceuticals – Advisory Committee/Board Member, Consultant. Bristol Myers Squibb – Advisory Committee/Board Member, Consultant. Eli Lilly – Advisory Committee/Board Member, Consultant. Gilead – Advisory Committee/Board Member, Consultant. Intellisphere (HCP Live Institutional Perspectives in GI) – Speakers Bureau. Janssen – Speakers Bureau. Janssen Biotech (nonbranded) – Consultant. Janssen Biotech (Women in GI Advisory Board) – Advisory Committee/Board Member, Consultant. Janssen Biotech NART – Consultant. Janssen Global Services – Consultant. Janssen Ortho – Advisory Committee/Board Member, Consultant. Microba – Advisory Committee/Board Member, Consultant. Pfizer – Grant/Research Support. Prime CME – Speakers Bureau. Prometheus Biosciences – Advisory Committee/Board Member, Consultant, Grant/Research Support. Takeda – Grant/Research Support, Speakers Bureau. UCB Biopharma – Advisory Committee/Board Member, Consultant. WebMD Global – Advisory Committee/Board Member, Consultant.
Silvio Danese: AbbVie – Consultant, Speakers Bureau. Allergan – Consultant, Speakers Bureau. Amgen – Consultant, Speakers Bureau. AstraZeneca – Consultant, Speakers Bureau. Athos Therapeutics – Consultant, Speakers Bureau. Biogen – Consultant, Speakers Bureau. Boehringer Ingelheim – Consultant, Speakers Bureau. Celgene – Consultant, Speakers Bureau. Celltrion – Consultant, Speakers Bureau. Eli Lilly – Consultant, Speakers Bureau. Enthera – Consultant, Speakers Bureau. Ferring – Consultant, Speakers Bureau. Gilead – Consultant, Speakers Bureau. Hospira – Consultant, Speakers Bureau. Inotrem – Consultant, Speakers Bureau. Janssen – Consultant, Speakers Bureau. Johnson & Johnson – Consultant, Speakers Bureau. MSD – Consultant, Speakers Bureau. Mundipharma – Consultant, Speakers Bureau. Mylan – Consultant, Speakers Bureau. Pfizer – Consultant, Speakers Bureau. Roche – Consultant, Speakers Bureau. Sandoz – Consultant, Speakers Bureau. Sublimity Therapeutics – Consultant, Speakers Bureau. Takeda – Consultant, Lecture fees. TiGenix – Consultant, Speakers Bureau. UCB Inc – Consultant, Speakers Bureau. Vifor – Consultant, Speakers Bureau.
William Sandborn: AbbVie – Consultant, Grant/Research Support. Abivax – Consultant. Admirx – Consultant. Alfa sigma – Consultant. Alimentiv (previously Robarts Clinical Trials, owned by Alimentiv Health Trust) – Consultant. Alivio Therapeutics – Consultant. Allakos – Consultant, Stock Options. Allergan – Consultant. Amgen – Consultant. Applied Molecular Transport – Consultant. Arena Pharmaceuticals – Consultant, Grant/Research Support. Avexegen Therapeutics – Consultant. Aviva – Grant/Research Support. Bausch Health (Salix) – Consultant. BeiGene – Consultant, Stock Options. Bellatrix Pharmaceuticals – Consultant. Boehringer Ingelheim – Consultant, Grant/Research Support. Boston Pharmaceuticals – Consultant. Bristol Myers Squibb – Consultant. Celgene – Consultant, Grant/Research Support. Celltrion – Consultant. Cellularity – Consultant. Conatus – Consultant. Cosmo Pharmaceuticals – Consultant. Equillium – Consultant. Escalier Biosciences – Consultant. Ferring – Consultant. Forbion – Consultant. Genentech – Grant/Research Support. Genentech/Roche – Consultant. Gilead Sciences – Consultant, Grant/Research Support. Glenmark Pharmaceuticals – Consultant. Gossamer Bio – Consultant, Stock Options. GSK – Grant/Research Support. Immunic (Vital Therapies) – Consultant. Incyte – Consultant. Index Pharmaceuticals – Consultant. Intact Therapeutics – Consultant. Janssen – Consultant, Grant/Research Support. Kyowa Kirin Pharmaceutical Research – Consultant. Kyverna Therapeutics – Consultant. Landos Biopharma – Consultant. Lilly – Consultant, Grant/Research Support. Miraca Life Sciences – Consultant. Nivalis Therapeutics – Consultant. Novartis – Consultant. Nutrition Science Partners – Consultant. Oppilan Pharma (acquired by Ventyx Biosciences) – Consultant, Stock Options. Otsuka – Consultant. Pandion Therapeutics – Consultant. Paul Hastings – Consultant. Pfizer – Consultant, Grant/Research Support. Progenity – Consultant, Stock Options. Prometheus Biosciences – Consultant, Grant/Research Support, Stock Options. Prometheus Laboratories – Consultant, Stock Options. Protagonist Therapeutics – Consultant. Provention Bio – Consultant. Reistone Biopharma – Consultant. Ritter Pharmaceuticals – Consultant. Seres Therapeutics – Consultant, Grant/Research Support. Shanghai Pharma Biotherapeutics – Consultant. Shire – Consultant, Grant/Research Support. Shoreline Biosciences – Consultant, Employee, Stock Options. Sienna Biopharmaceuticals – Consultant. Sigmoid Biotechnologies – Consultant. Sterna Biologicals – Consultant. Sublimity Therapeutics – Consultant. Surrozen – Consultant. Takeda – Consultant, Grant/Research Support. TheraVance Biopharma – Consultant, Grant/Research Support. Thetis Pharmaceuticals – Consultant. Tigenix – Consultant. Tillotts Pharma – Consultant. UCB Pharma – Consultant. Vendata Biosciences – Consultant. Ventyx Biosciences – Consultant, Stock Options. Vimalan Biosciences – Consultant, Stock Options. Vivelix Pharmaceuticals – Consultant. Vivreon Biosciences – Consultant, Stock Options. Zealand Pharma – Consultant.
Ye Miao: Janssen Research & Development, LLC – Employee, Stock-publicly held company(excluding mutual/index funds).
Hongyan Zhang: Johnson & Johnson – Employee, Stock-publicly held company(excluding mutual/index funds).
Remo Panaccione: Abbott – Consultant. AbbVie – Advisory Committee/Board Member, Consultant, Grant/Research Support, Speakers Bureau. Alimentiv – Consultant. Amgen – Advisory Committee/Board Member, Consultant. Arena – Advisory Committee/Board Member, Consultant, Speakers Bureau. AstraZeneca – Consultant. Boehringer Ingelheim – Consultant. Bristol Myers Squibb – Advisory Committee/Board Member, Consultant. Celgene – Advisory Committee/Board Member, Consultant, Speakers Bureau. Celltrion – Advisory Committee/Board Member, Consultant. Cosmos Pharmaceuticals – Consultant. Eisai – Consultant. Elan – Consultant. Eli Lilly – Advisory Committee/Board Member, Consultant, Speakers Bureau. Ferring – Advisory Committee/Board Member, Consultant, Grant/Research Support, Speakers Bureau. Galapagos – Advisory Committee/Board Member, Consultant. Genentech – Advisory Committee/Board Member, Consultant. Gilead Sciences – Advisory Committee/Board Member, Consultant, Speakers Bureau. GlaxoSmithKline – Advisory Committee/Board Member, Consultant. Janssen – Advisory Committee/Board Member, Consultant, Grant/Research Support, Speakers Bureau. Merck – Advisory Committee/Board Member, Consultant, Speakers Bureau. Mylan – Advisory Committee/Board Member, Consultant. Oppilan Pharma – Advisory Committee/Board Member, Consultant. Pandion Pharma – Advisory Committee/Board Member. Pandion Therapeutics – Consultant. Pfizer – Advisory Committee/Board Member, Consultant, Grant/Research Support, Speakers Bureau. Progenity – Consultant. Protagonist Therapeutics – Consultant. Roche – Consultant, Speakers Bureau. Sandoz – Advisory Committee/Board Member, Consultant, Speakers Bureau. Satisfai Health – Consultant. Schering-Plough – Consultant. Shire – Advisory Committee/Board Member, Consultant, Speakers Bureau. Sublimity Therapeutics – Advisory Committee/Board Member, Consultant. Takeda – Advisory Committee/Board Member, Consultant, Grant/Research Support, Speakers Bureau. Theravance – Advisory Committee/Board Member, Consultant. UCB – Consultant.
Tadakazu Hisamatsu: AbbVie – Consultant, Grant/Research Support, Lecture fees. Celgene – Consultant. Daiichi-Sankyo – Grant/Research Support. EA Pharma Co, Ltd – Consultant, Grant/Research Support, Lecture fees. Janssen Research & Development, LLC – Consultant, lecture fees. JIMRO – Grant/Research Support. JIMRO Co – Consultant, Grant/Research Support. Kyorin Pharmaceutical Co. Ltd – Consultant, Grant/Research Support. Mitsubishi Tanabe Pharma Corporation – Grant/Research Support, Lecture fees. Mochida Pharmaceutical Co., Ltd – Grant/Research Support, lecture fees. Nichi-Iko Pharmaceutical Co., Ltd – Consultant. Nippon Kayaku Co., Ltd – Grant/Research Support. Pfizer Inc – Consultant, Grant/Research Support, lecture fees. Takeda Pharmaceutical Co., Ltd – Grant/Research Support, Lecture fees. Zeria Pharmaceutical Co. Ltd – Grant/Research Support.
Ellen Scherl: AbbVie – Consultant, Grant/Research Support. AstraZeneca – Grant/Research Support. Bristol Myers Squibb – Consultant. Celgene – Grant/Research Support. Crohn’s and Colitis Foundation – Consultant, Grant/Research Support. Entera Health – Consultant. Evidera – Consultant. Genentech – Grant/Research Support. GI Health Foundation – Consultant, Speakers Bureau. Gilead – Stock Options. Janssen Research & Development, LLC – Consultant, Grant/Research Support. Johns Hopkins University – Grant/Research Support. National Institute of Diabetes and Digestive and Kidney – Grant/Research Support. National Institute of Health – Grant/Research Support. New York Crohn’s Foundation – Grant/Research Support. Pfizer – Grant/Research Support. Protagonist – Consultant. Seres – Consultant, Grant/Research Support. Takeda – Consultant. UCB – Grant/Research Support.
Rupert Leong: AbbVie – Consultant. Aspen – Consultant. Bristol Myers Squibb – Consultant. Celgene – Consultant. Chiesi – Consultant. Dr Falk Pharma – Consultant. Endochoice – Grant/Research Support. Ferring – Consultant, Grant/Research Support. GESA – Grant/Research Support. Glutagen – Consultant. Gutsy Group – Grant/Research Support. Hospira – Consultant. Janssen – Consultant, Grant/Research Support. MSD – Consultant. NHMRC – Grant/Research Support. Novartis – Consultant. Pfizer – Consultant, Grant/Research Support. Shire – Grant/Research Support. Takeda – Consultant, Grant/Research Support.
David Rowbotham: AbbVie – Advisory Committee/Board Member, Speakers Bureau. Baxter – Advisory Committee/Board Member, Speakers Bureau. bioCSL – Speakers Bureau. Emerge Health – Speakers Bureau. Given Imaging – Speakers Bureau. Hospira – Advisory Committee/Board Member. Janssen Research & Development, LLC – Advisory Committee/Board Member, Speakers Bureau. Pharmaco – Advisory Committee/Board Member.
Ramesh Arasaradnam: AbbVie – Consultant, Grant/Research Support. Dr Falk Pharma – Consultant, Grant/Research Support. Janssen Research & Development, LLC – Consultant, Grant/Research Support.
Laurent Peyrin-Biroulet: AbbVie – Advisory Committee/Board Member, Consultant, Speakers Bureau. Amgen – Advisory Committee/Board Member, Consultant, Speakers Bureau. Biogaran – Advisory Committee/Board Member, Consultant, Speakers Bureau. Biogen – Advisory Committee/Board Member, Consultant, Speakers Bureau. Boehringer Ingelheim – Advisory Committee/Board Member, Consultant, Speakers Bureau. Celgene – Advisory Committee/Board Member, Consultant, Speakers Bureau. Celltrion – Advisory Committee/Board Member, Consultant, Speakers Bureau. Eli Lilly – Advisory Committee/Board Member, Consultant, Speakers Bureau. Ferring – Advisory Committee/Board Member, Consultant, Speakers Bureau. Forward Pharma – Advisory Committee/Board Member, Consultant, Speakers Bureau. Genentech – Advisory Committee/Board Member, Consultant, Speakers Bureau. H.A.C. Pharma – Advisory Committee/Board Member, Consultant, Speakers Bureau. Hospira/Pfizer – Advisory Committee/Board Member, Consultant, Speakers Bureau. Index Pharmaceuticals – Advisory Committee/Board Member, Consultant, Speakers Bureau. Janssen – Advisory Committee/Board Member, Consultant, Speakers Bureau. Lycera – Advisory Committee/Board Member, Consultant, Speakers Bureau. Merck – Advisory Committee/Board Member, Consultant, Speakers Bureau. Mitsubishi – Advisory Committee/Board Member, Consultant, Speakers Bureau. Norgine – Advisory Committee/Board Member, Consultant, Speakers Bureau. Samsung Bioepis – Advisory Committee/Board Member, Consultant, Speakers Bureau. Sandoz – Advisory Committee/Board Member, Consultant, Speakers Bureau. Takeda – Advisory Committee/Board Member, Consultant, Speakers Bureau. Theravance – Advisory Committee/Board Member, Consultant, Speakers Bureau. Tillotts – Advisory Committee/Board Member, Consultant, Speakers Bureau. Vifor – Advisory Committee/Board Member, Consultant, Speakers Bureau.
Waqqas Afif: Janssen Research & Development, LLC – Grant/Research Support.
Colleen Marano: Janssen Research & Development, LLC – Employee, Stock-publicly held company(excluding mutual/index funds).
Bruce E. Sands, MD, MS, FACG1, Maria T. Abreu, MD2, Silvio Danese, MD, PhD3, William J. Sandborn, MD4, Ye Miao, MS5, Hongyan Zhang, PhD5, Remo Panaccione, MD6, Tadakazu Hisamatsu, MD, PhD7, Ellen J. Scherl, MD8, Rupert W. Leong, MD9, David S. Rowbotham, MD10, Ramesh P. Arasaradnam, PhD11, Laurent Peyrin-Biroulet, MD, PhD12, Waqqas Afif, MD13, Colleen Marano, PhD5. A0394 - Efficacy and Safety of Ustekinumab for Ulcerative Colitis Through 4 Years: Final Results From the UNIFI Long-Term Extension, ACG 2022 Annual Scientific Meeting Abstracts. Charlotte, NC: American College of Gastroenterology.
