Back

Poster Session A - Sunday Afternoon
Category: IBD
A0395 - QUASAR Induction Study 1 Cumulative Response to Guselkumab in Patients With Moderately to Severely Active Ulcerative Colitis
Sunday, October 23, 2022
5:00 PM – 7:00 PM ET
Location: Crown Ballroom

Has Audio

Bruce E. Sands, MD, MS, FACG
Icahn School of Medicine at Mount Sinai
New York, NY
Presenting Author(s)
Bruce E. Sands, MD, MS, FACG1, David T. Rubin, MD, FACG2, Gary Lichtenstein, MD3, Nicole Shipitofsky, PharmD4, Kuan-Hsiang Huang, MD, PhD4, Matthew Germinaro, MD5, Rebbecca Wilson, DPH4, Hongyan Zhang, PhD4, Aaron DuVall, MD6, Qian Cao, PhD7, Jessica R. Allegretti, MD, MPH8, Brian G. Feagan, MD9, Laurent Peyrin-Biroulet, MD, PhD10, Tadakazu Hisamatsu, MD, PhD11, Julian Panés, MD12, Axel Dignass, MD, PhD13, Brian Bressler, MD, MS14
1Icahn School of Medicine at Mount Sinai, New York, NY; 2University of Chicago Medicine Inflammatory Bowel Disease Center, Chicago, IL; 3University of Pennsylvania, Philadelphia, PA; 4Janssen Research & Development, LLC, Spring House, PA; 5Janssen Research & Development, LLC., Spring House, PA; 6Tyler Research Institute, LLC, Tyler, TX; 7Zhejiang University School of Medicine, Hangzhou, Zhejiang, China; 8Brigham and Women’s Hospital Crohn’s and Colitis Center, Boston, MA; 9Alimentiv, Inc.; Western University, London, ON, Canada; 10University of Lorraine, Nancy, Lorraine, France; 11Kyorin University School of Medicine, Tokyo, Tokyo, Japan; 12Hospital Clínic de Barcelona, IDIBAPS, CIBERehd, Barcelona, Catalonia, Spain; 13Agaplesion Markus Hospital / Goethe University, Frankfurt, Hessen, Germany; 14University of British Columbia, Vancouver, BC, Canada
Introduction: Guselkumab (GUS), an IL-23p19 antagonist, had greater efficacy than placebo (PBO) in achieving clinical response and clinical remission at Week (Wk) 12 in the randomized, controlled Phase 2b QUASAR Induction Study 1 (NCT04033445) in patients with moderately to severely active ulcerative colitis (UC).1 Patients who were not in clinical response at Wk 12 received GUS treatment through Wk 24. Here, we report GUS cumulative efficacy and safety results for Induction Study 1.
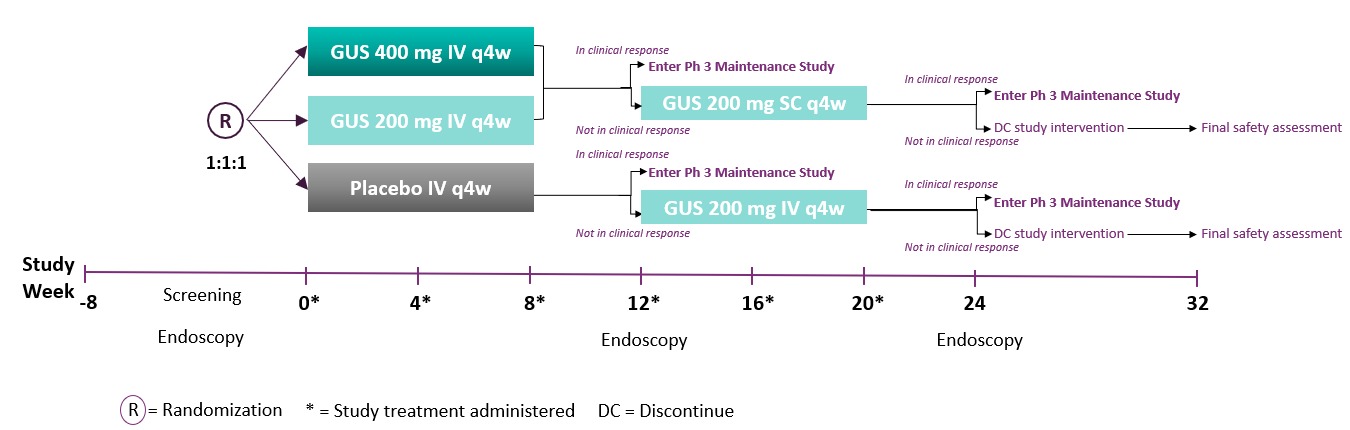
Methods: Eligible patients had moderately to severely active UC (modified Mayo score of 5 to 9 with a Mayo endoscopy subscore ≥2) at baseline. Patients were randomized 1:1:1 to IV GUS 200mg, 400mg, or PBO at Wks 0, 4, and 8. Patients who were not in clinical response to IV induction at Wk 12 received GUS treatment (PBO IV→GUS 200mg IV; GUS 200mg IV→GUS 200mg SC; GUS 400mg IV→GUS 200mg SC) at Wks 12, 16, and 20 and were evaluated at Wk 24 (Figure 1). Matching IV or SC PBO was administered to maintain the blind.
Results: Three hundred thirteen patients were randomized and treated at baseline. Demographic and disease characteristics at baseline were similar among the treatment groups, and approximately 50% had a prior inadequate response or intolerance to advanced UC therapy.
At Wk 12, clinical response was achieved by 61.4% (62/101) and 60.7% (65/107) of patients randomized to GUS 200mg and GUS 400mg IV vs 27.6 % (29/105) of patients randomized to PBO IV (both p< 0.001). Of the patients in the GUS groups who were not in clinical response at Wk 12, 54.3% (19/35) in the GUS 200mg IV→200mg SC group and 50.0% (19/38) in the GUS 400mg IV→200mg SC group achieved clinical response at Wk 24. Clinical response at Wk 12 or 24 was achieved by 80.2% of patients who were randomized to GUS 200mg IV and 78.5% of patients who were randomized to GUS 400mg IV. For patients who received PBO IV→GUS 200mg IV, clinical response at Wk 24 (65.2%) was similar to Wk 12 clinical response following GUS 200mg IV induction (61.4%). The most frequent adverse events among all GUS-treated pts (n=274) were anemia (7.7%), headache (5.1%), worsening UC (4.4%), COVID-19 (3.6%), arthralgia (2.9%) and abdominal pain (2.6%) which are consistent with Wk 12 results.
Discussion: Overall, approximately 80% of patients randomized to receive GUS achieved clinical response at Wk 12 or 24. Continued treatment with SC GUS allowed 50-54.3% of IV GUS Wk 12 clinical nonresponders to achieve clinical response at Wk 24. No new safety concerns for GUS were identified.
Disclosures:
Bruce E. Sands, MD, MS, FACG1, David T. Rubin, MD, FACG2, Gary Lichtenstein, MD3, Nicole Shipitofsky, PharmD4, Kuan-Hsiang Huang, MD, PhD4, Matthew Germinaro, MD5, Rebbecca Wilson, DPH4, Hongyan Zhang, PhD4, Aaron DuVall, MD6, Qian Cao, PhD7, Jessica R. Allegretti, MD, MPH8, Brian G. Feagan, MD9, Laurent Peyrin-Biroulet, MD, PhD10, Tadakazu Hisamatsu, MD, PhD11, Julian Panés, MD12, Axel Dignass, MD, PhD13, Brian Bressler, MD, MS14. A0395 - QUASAR Induction Study 1 Cumulative Response to Guselkumab in Patients With Moderately to Severely Active Ulcerative Colitis, ACG 2022 Annual Scientific Meeting Abstracts. Charlotte, NC: American College of Gastroenterology.
1Icahn School of Medicine at Mount Sinai, New York, NY; 2University of Chicago Medicine Inflammatory Bowel Disease Center, Chicago, IL; 3University of Pennsylvania, Philadelphia, PA; 4Janssen Research & Development, LLC, Spring House, PA; 5Janssen Research & Development, LLC., Spring House, PA; 6Tyler Research Institute, LLC, Tyler, TX; 7Zhejiang University School of Medicine, Hangzhou, Zhejiang, China; 8Brigham and Women’s Hospital Crohn’s and Colitis Center, Boston, MA; 9Alimentiv, Inc.; Western University, London, ON, Canada; 10University of Lorraine, Nancy, Lorraine, France; 11Kyorin University School of Medicine, Tokyo, Tokyo, Japan; 12Hospital Clínic de Barcelona, IDIBAPS, CIBERehd, Barcelona, Catalonia, Spain; 13Agaplesion Markus Hospital / Goethe University, Frankfurt, Hessen, Germany; 14University of British Columbia, Vancouver, BC, Canada
Introduction: Guselkumab (GUS), an IL-23p19 antagonist, had greater efficacy than placebo (PBO) in achieving clinical response and clinical remission at Week (Wk) 12 in the randomized, controlled Phase 2b QUASAR Induction Study 1 (NCT04033445) in patients with moderately to severely active ulcerative colitis (UC).1 Patients who were not in clinical response at Wk 12 received GUS treatment through Wk 24. Here, we report GUS cumulative efficacy and safety results for Induction Study 1.
Methods: Eligible patients had moderately to severely active UC (modified Mayo score of 5 to 9 with a Mayo endoscopy subscore ≥2) at baseline. Patients were randomized 1:1:1 to IV GUS 200mg, 400mg, or PBO at Wks 0, 4, and 8. Patients who were not in clinical response to IV induction at Wk 12 received GUS treatment (PBO IV→GUS 200mg IV; GUS 200mg IV→GUS 200mg SC; GUS 400mg IV→GUS 200mg SC) at Wks 12, 16, and 20 and were evaluated at Wk 24 (Figure 1). Matching IV or SC PBO was administered to maintain the blind.
Results: Three hundred thirteen patients were randomized and treated at baseline. Demographic and disease characteristics at baseline were similar among the treatment groups, and approximately 50% had a prior inadequate response or intolerance to advanced UC therapy.
At Wk 12, clinical response was achieved by 61.4% (62/101) and 60.7% (65/107) of patients randomized to GUS 200mg and GUS 400mg IV vs 27.6 % (29/105) of patients randomized to PBO IV (both p< 0.001). Of the patients in the GUS groups who were not in clinical response at Wk 12, 54.3% (19/35) in the GUS 200mg IV→200mg SC group and 50.0% (19/38) in the GUS 400mg IV→200mg SC group achieved clinical response at Wk 24. Clinical response at Wk 12 or 24 was achieved by 80.2% of patients who were randomized to GUS 200mg IV and 78.5% of patients who were randomized to GUS 400mg IV. For patients who received PBO IV→GUS 200mg IV, clinical response at Wk 24 (65.2%) was similar to Wk 12 clinical response following GUS 200mg IV induction (61.4%). The most frequent adverse events among all GUS-treated pts (n=274) were anemia (7.7%), headache (5.1%), worsening UC (4.4%), COVID-19 (3.6%), arthralgia (2.9%) and abdominal pain (2.6%) which are consistent with Wk 12 results.
Discussion: Overall, approximately 80% of patients randomized to receive GUS achieved clinical response at Wk 12 or 24. Continued treatment with SC GUS allowed 50-54.3% of IV GUS Wk 12 clinical nonresponders to achieve clinical response at Wk 24. No new safety concerns for GUS were identified.

Figure: Figure 1. QUASAR Induction Study 1: Study Design
Disclosures:
Bruce Sands: Abivax – Consultant, Speaking. Amgen – Consultant. Arena Pharmaceuticals – Consultant. Artugen Therapeutics – Consultant. AstraZeneca – Consultant. Bacainn Therapeutics – Consultant. Boehringer-Ingelheim – Consultant. Boston Pharmaceuticals – Consultant. Bristol Myers Squibb – Consultant, speaking, research funding. Calibr – Consultant. Celltrion Healthcare – Consultant. ClostraBio – Consultant. Eli Lilly and Company – Consultant. Entera – Consultant. Evommune – Consultant. Galapagos – Consultant. Genentech – Consultant. Gilead Sciences – Consultant. GlaxoSmithKline – Consultant. Gossamer Bio – Consultant. InDex Pharmaceuticals – Consultant. Innovation Therapeutics – Consultant. Inotrem – Consultant. Ironwood Pharmaceuticals – Consultant. Janssen – Consultant, Grant/Research Support, speaking. Kaleido – Consultant. Kallyope – Consultant. Miro Bio – Consultant. Morphic Therapeutics – Consultant. MRM Health – Consultant. Pfizer – Consultant, speaking. Progenity – Consultant. Prometheus Biosciences – Consultant. Protagonist Therapeutics – Consultant. Q32 Bio – Consultant. Surrozen – Consultant. Takeda – Consultant, Speaking. Teva – Consultant. TLL Pharmaceutical – Consultant. USWM Enterprises – Consultant. VielaBio – Consultant.
David Rubin: AbbVie – Consultant. Alimentiv Inc. – Consultant. AltruBio – Consultant. Arena Pharmaceuticals – Consultant. Aslan Pharmaceuticals – Consultant. Athos Therapeutics – Consultant. Bellatrix Pharmaceuticals – Consultant. Boehringer Ingelheim, Ltd. – Consultant. Bristol Myers Squibb – Consultant. Celgene Corp/Syneos – Consultant. ClostraBio – Consultant. Connect BioPharma – Consultant. EcoR1 – Consultant. Genentech/Roche – Consultant. Gilead Sciences – Consultant. Ironwood Pharmaceuticals – Consultant. Iterative Scopes – Consultant. Janssen – Consultant. Kaleido Biosciences – Consultant. Lilly, Eli & Co – Consultant. Materia Prima – Consultant. Pfizer – Consultant. Prometheus Biosciences – Consultant. Reistone Biopharma – Consultant. Seres Therapeutics, Inc – Consultant. Takeda – Consultant, Grant/Research Support. Target RWE – Consultant. Techlab – Consultant. Trellus Health – Consultant.
Gary Lichtenstein: Abbvie – Consultant. Allergan – Consultant. American College of Gastroenterology – Honorarium for Associate Editor of American Journal of Gastroenterology. American Gastroenterological Association – CME. American Regent – Consultant, Honorarium (CME Program). Celgene – Consultant, Grant/Research Support. Chemed – CME. Eli Lilly – Consultant, Data Safety Monitoring Board. Endo Pharmaceuticals – Consultant. Ferring – Consultant. Gastroenterology and Hepatology – Gastro-Hep Communication, Editor-Honorarium. Gilead – Consultant. IMEDEX – CME. Ironwood – CME. Janssen/ Janssen Orthobiotech – Consultant, Grant/Research Support, Funding to University of PA (IBD Fellow Education). MedEd Consultants – Consultant. Merck – Consultant, Honorarium (CME Program). Morphic Therapeutics – Consultant. Pfizer Pharmaceuticals – Consultant, Funding to University of PA (IBD Fellow Education). Professional Communications, Inc. – Royalty for writing Textbook. Prometheus Laboratories, Inc – Consultant. Romark – Consultant, Honorarium for CME. Salix Pharmaceuticals/Valeant – Consultant. Sandoz – Consultant. Shire Pharmaceuticals – Consultant. SLACK, Inc – Book Royalty. Springer Science and Business Media – Editor (Honorarium). Takeda – Consultant, Grant/Research Support, Funding to University of PA (IBD Fellow Education). UCB – Consultant, Grant/Research Support. University of Kentucky – CME. Up-To-Date – Author (Honorarium). Vindico – CME. Virgo – Consultant, Stock Options.
Nicole Shipitofsky: Janssen – Employee, Stock Options.
Kuan-Hsiang Huang: Johnson & Johnson – Employee, Stock-publicly held company(excluding mutual/index funds).
Matthew Germinaro: Janssen Research & Development – Employee. Johnson & Johnson – Stock-publicly held company(excluding mutual/index funds).
Rebbecca Wilson: Johnson & Johnson – Employee, Stock-publicly held company(excluding mutual/index funds).
Hongyan Zhang: Johnson & Johnson – Employee, Stock-publicly held company(excluding mutual/index funds).
Aaron DuVall: Janssen Research & Development, LLC – Consultant, Clinical investigator.
Qian Cao: Janssen Research & Development, LLC – Consultant, Clinical investigator.
Jessica Allegretti: Artugen – Consultant. Baccain – Consultant. Bristol Myers Squibb – Consultant, Speaker. Ferring Pharmaceuticals – Consultant. Finch Therapeutics – Consultant, Grant/Research Support. Iterative Scopes – Consultant. Janssen – Consultant, Grant/Research Support. Merck – Grant/Research Support. Morphic – Consultant. Pfizer – Consultant, Grant/Research Support. Seres Therapeutics – Consultant. Servatus – Consultant. Takeda – Consultant.
Brian Feagan: AbbVie – Consultant, Member of the scientific advisory board, Speakers Bureau. AbolerIS – Consultant. AgomAB Therapeutics – Consultant. Alimentiv Inc – Employee. Allianthera – Consultant. Amgen – Consultant, Member of the scientific advisory board. AnaptysBio – Consultant. Applied Molecular Transport Inc. – Consultant. Arena Pharma – Consultant. Avir – Consultant. Azora Therapeutics – Consultant. BioJamp – Consultant. Biopharma – Consultant. Biora Therapeutics – Consultant. Boehringer-Ingelheim – Consultant, Member of scientific advisory board. Boston Pharma – Consultant. Boxer – Consultant. Celgene/BMS – Consultant, Member of scientific advisory board. Connect BioPharma – Consultant. Cytoki – Consultant. Disc Medicine – Consultant. Duality – Consultant. EcoR1 Capital – Consultant, Member of the scientific advisory board. Eli Lilly and Company – Consultant. Equillium – Consultant. Ermium – Consultant. Everest Clinical Research Corp. – Consultant. Ferring – Consultant. First Wave – Consultant. Galapagos – Consultant. Galen Atlantica – Consultant. Genentech/Roche – Consultant, Member of scientific advisory board. Gilead – Consultant. Glenmark – Consultant. Gossamer Pharma – Consultant, Stock Shareholder. GSK – Consultant, Member of the scientific advisory board. Hoffmann-LaRoche – Consultant. Hot Spot Therapeutics – Consultant. Imhotex – Consultant. ImmunExt – Consultant. Immunic Therapeutics – Consultant. Index Pharma – Consultant, Member of the scientific advisory board. Intact Therapeutics – Consultant. JAKAcademy – Consultant. Janssen – Consultant, Member of scientific advisory board, Speakers Bureau. Japan Tobacco Inc. – Consultant. Kaleido Biosciences – Consultant. L.E.K. Consulting – Consultant. Landos Biopharma – Consultant. Leadiant – Consultant. LifeSci Capital – Consultant. Lument AB – Consultant. Millennium – Consultant. MiroBio – Consultant. Morphic Therapeutics – Consultant, Member of the scientific advisory board. Mylan – Consultant. Novartis – Member of the scientific advisory board. OM Pharma – Consultant. Origo BioPharma – Consultant, Member of scientific advisory board. Orphagen – Consultant. Otsuka – Consultant. Pandion Therapeutics – Consultant. Pfizer – Consultant, Member of the scientific advisory board. Play to Know AG – Consultant. Progenity – Consultant, Member of the scientific advisory board. Prometheus – Member of scientific advisory board. Prometheus Therapeutics and Diagnostics – Consultant. Protagonist – Consultant. PTM Therapeutics – Consultant. Q32 Bio – Consultant. Rebiotix – Consultant. RedHill – Consultant. REDX – Consultant. Roche – Consultant. Sandoz – Consultant. Sanofi – Consultant. Seres Therapeutics – Consultant. Silverback Therapeutics – Consultant. Surrozen Inc. – Consultant. Takeda – Consultant, Member of the scientific advisory board, Speakers Bureau. Teva – Consultant, Member of the scientific advisory board. Thelium – Consultant. Theravance – Consultant. Tigenix – Consultant. Tillotts Pharma – Consultant, Member of the scientific advisory board. UCB Pharma – Consultant. VHsquared Ltd. – Consultant. Viatris – Consultant. Western University – Employee. Ysios – Consultant. Ysopia – Consultant. Zealand Pharma – Consultant.
Laurent Peyrin-Biroulet: AbbVie – Advisory Committee/Board Member, Consultant, Speakers Bureau. Amgen – Advisory Committee/Board Member, Consultant, Speakers Bureau. Biogaran – Advisory Committee/Board Member, Consultant, Speakers Bureau. Biogen – Advisory Committee/Board Member, Consultant, Speakers Bureau. Boehringer Ingelheim – Advisory Committee/Board Member, Consultant, Speakers Bureau. Celgene – Advisory Committee/Board Member, Consultant, Speakers Bureau. Celltrion – Advisory Committee/Board Member, Consultant, Speakers Bureau. Eli Lilly – Advisory Committee/Board Member, Consultant, Speakers Bureau. Ferring – Advisory Committee/Board Member, Consultant, Speakers Bureau. Forward Pharma – Advisory Committee/Board Member, Consultant, Speakers Bureau. Genentech – Advisory Committee/Board Member, Consultant, Speakers Bureau. H.A.C. Pharma – Advisory Committee/Board Member, Consultant, Speakers Bureau. Hospira/Pfizer – Advisory Committee/Board Member, Consultant, Speakers Bureau. Index Pharmaceuticals – Advisory Committee/Board Member, Consultant, Speakers Bureau. Janssen – Advisory Committee/Board Member, Consultant, Speakers Bureau. Lycera – Advisory Committee/Board Member, Consultant, Speakers Bureau. Merck – Advisory Committee/Board Member, Consultant, Speakers Bureau. Mitsubishi – Advisory Committee/Board Member, Consultant, Speakers Bureau. Norgine – Advisory Committee/Board Member, Consultant, Speakers Bureau. Samsung Bioepis – Advisory Committee/Board Member, Consultant, Speakers Bureau. Sandoz – Advisory Committee/Board Member, Consultant, Speakers Bureau. Takeda – Advisory Committee/Board Member, Consultant, Speakers Bureau. Theravance – Advisory Committee/Board Member, Consultant, Speakers Bureau. Tillotts – Advisory Committee/Board Member, Consultant, Speakers Bureau. Vifor – Advisory Committee/Board Member, Consultant, Speakers Bureau.
Tadakazu Hisamatsu: AbbVie – Consultant, Grant/Research Support, Lecture fees. Celgene – Consultant. Daiichi-Sankyo – Grant/Research Support. EA Pharma Co, Ltd – Consultant, Grant/Research Support, Lecture fees. Janssen Research & Development, LLC – Consultant, lecture fees. JIMRO – Grant/Research Support. JIMRO Co – Consultant, Grant/Research Support. Kyorin Pharmaceutical Co. Ltd – Consultant, Grant/Research Support. Mitsubishi Tanabe Pharma Corporation – Grant/Research Support, Lecture fees. Mochida Pharmaceutical Co., Ltd – Grant/Research Support, lecture fees. Nichi-Iko Pharmaceutical Co., Ltd – Consultant. Nippon Kayaku Co., Ltd – Grant/Research Support. Pfizer Inc – Consultant, Grant/Research Support, lecture fees. Takeda Pharmaceutical Co., Ltd – Grant/Research Support, Lecture fees. Zeria Pharmaceutical Co. Ltd – Grant/Research Support.
Julian Panés: Abbott – payment for the development of educational presentations, Speakers Bureau. AbbVie – Consultant, Grant/Research Support, support for travel to meetings, during the conduct of the study; payment for development of educational presentations. Arena – Consultant. Athos – Consultant. Boehringer Ingelheim – Consultant. Celgene – Consultant. Celltrion – Consultant. Ferring – Consultant. Galapagos – Consultant. Genentech-Roche – Consultant. GSK – Consultant. Janssen – Consultant, Payment for development of educational presentations, Speakers Bureau. Mirum – Consultant. Morphic – Consultant. MSD – Consultant. Nestlé – Consultant. Origo – Consultant. Pandion – Consultant. Pfizer – Consultant, Grant/Research Support, Payment for development of educational presentations, Speakers Bureau. Progenity – Consultant. Protagonist – Consultant. Revolo – Consultant. Robarts – Consultant. Roche – Payment for development of educational presentations. Takeda – Consultant, Support for travel to meetings during the conduct of the study, Speakers Bureau. Theravance – Consultant, Speakers Bureau. Wassermann – Consultant.
Axel Dignass: AbbVie – Consultant, Speakers Bureau. Abivax – Consultant. Amgen – Consultant. Biogen – Consultant. Boehringer Ingelheim – Consultant. Celgene/Bristol Myers Squibb – Consultant. Eli Lilly – Consultant, Speakers Bureau. Falk Foundation – Consultant, Speakers Bureau. Ferring – Consultant, Speakers Bureau. Fresenius Kabi – Consultant. Galapagos – Consultant, Speakers Bureau. Gilead – Consultant, Speakers Bureau. Janssen – Consultant, Speakers Bureau. MSD – Consultant, Speakers Bureau. Pfizer – Consultant, Speakers Bureau. Pharmacosmos – Consultant. Roche/Genentech – Consultant. Sandoz/Hexal – Consultant. Takeda – Consultant, Speakers Bureau. Tillotts – Consultant, Speakers Bureau. Vifor – Consultant, Speakers Bureau.
Brian Bressler: Abbvie – Advisor or Review Panel Member, Grant/Research Support, Speakers Bureau. Allergan – Advisor or Review Panel Member. Alvine – Grant/Research Support. Amgen – Advisor or Review Panel Member, Grant/Research Support. Boehringer Ingelheim – Grant/Research Support. Bristol Myers Squibb – Advisor or Review Panel Member, Grant/Research Support. Celgene – Advisor or Review Panel Member, Grant/Research Support. Ferring – Advisor or Review Panel Member, Speakers Bureau. Genentech – Advisor or Review Panel Member, Grant/Research Support. GSK – Grant/Research Support. Janssen – Advisor or Review Panel Member, Grant/Research Support, Speakers Bureau. Merck – Advisor or Review Panel Member, Grant/Research Support, Speakers Bureau. Microbiome Insights – Advisor or Review Panel Member. Novartis – Advisor or Review Panel Member, Speakers Bureau. Pendopharm – Advisor or Review Panel Member. Pfizer – Advisor or Review Panel Member, Speakers Bureau. Protagonist – Advisor or Review Panel Member. Qu Biologic – Grant/Research Support, Stock Options. Robarts Clinical Trials – Advisor or Review Panel Member. Takeda – Advisor or Review Panel Member, Speakers Bureau.
Bruce E. Sands, MD, MS, FACG1, David T. Rubin, MD, FACG2, Gary Lichtenstein, MD3, Nicole Shipitofsky, PharmD4, Kuan-Hsiang Huang, MD, PhD4, Matthew Germinaro, MD5, Rebbecca Wilson, DPH4, Hongyan Zhang, PhD4, Aaron DuVall, MD6, Qian Cao, PhD7, Jessica R. Allegretti, MD, MPH8, Brian G. Feagan, MD9, Laurent Peyrin-Biroulet, MD, PhD10, Tadakazu Hisamatsu, MD, PhD11, Julian Panés, MD12, Axel Dignass, MD, PhD13, Brian Bressler, MD, MS14. A0395 - QUASAR Induction Study 1 Cumulative Response to Guselkumab in Patients With Moderately to Severely Active Ulcerative Colitis, ACG 2022 Annual Scientific Meeting Abstracts. Charlotte, NC: American College of Gastroenterology.
