Back

Poster Session B - Monday Morning
Category: Biliary/Pancreas
B0031 - Type 1 Autoimmune Pancreatitis Unmasked by COVID-19 Vaccine
Monday, October 24, 2022
10:00 AM – 12:00 PM ET
Location: Crown Ballroom

Has Audio
- EB
Erica C. Becker, MD, MPH
University of Connecticut Health Center
Farmington, CT
Presenting Author(s)
Erica C. Becker, MD, MPH1, Osama Siddique, MD2, Dinesh Kapur, MD3, Krishna Patel, MD2, Vaibhav Mehendiratta, MD2
1University of Connecticut Health Center, Farmington, CT; 2Hartford Hospital, Hartford, CT; 3William W. Backus Hospital, Norwich, CT
Introduction: Type 1 AIP is a rare form of idiopathic chronic pancreatitis associated with IgG4-related systemic disease. It predominantly affects male adults and has multi-organ extra-pancreatic manifestations. It is much less associated with inflammatory bowel disease compared to type 2 AIP, which is reported to have a least a 15% predilection for ulcerative colitis. To date there are no reports of vaccine-induced type 1 AIP. We present a case of newly diagnosed type 1 AIP in a patient one month after receiving COVID-19 vaccination.
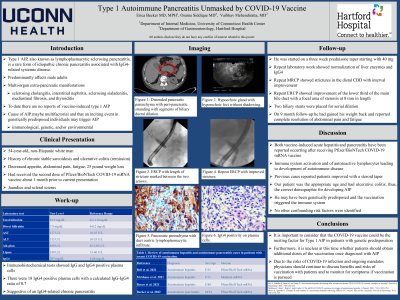
Case Description/Methods: 54-year-old male with history of UC presented with decreased appetite, abdominal pain, fatigue, and 25 pound unintentional weight loss. He had received the second dose of Pfizer/BioNTech COVID-19 mRNA vaccine about 1 month prior. Laboratory tests were significant for hypovolemia, hyperglycemia, and elevated liver enzymes. IgG4 level was 287.0 mg/dL. CT scan showed distended pancreatic parenchyma with peri-pancreatic stranding with segments of biliary ductal dilation. MRCP showed dilated bile ducts to the level of the head of the pancreas where there was an abrupt truncation of the extrahepatic common duct. The pancreas was diffusely enlarged with some irregular beading and narrowing of the main pancreatic duct. On ERCP the lower third of the main bile duct contained a single severe stenosis 20-25 mm in length and a biliary stent was placed. EUS was performed and pancreatic parenchyma was diffusely abnormal with lobularity, generalized hypoechoic gland, hyperechoic foci without shadowing. The pancreatic duct was irregular in contour. Immunohistochemical tests showed IgG and IgG4 positive plasma cells. The patient was started on a three week prednisone taper. On 9 month follow-up clinical symptoms, laboratory work and repeat imaging showed improvement.
Discussion: Both vaccine-induced acute hepatitis and pancreatitis have been reported occurring 2-3 days after receiving Pfizer/BioNTech COVID-19 mRNA vaccine. Our case suggests a temporal association between COVID-19 vaccination and AIP although a cause effect relation cannot be definitely established. Our patient was the appropriate age and had ulcerative colitis; thus, the correct demographic for developing AIP. He may have been genetically predisposed and the vaccination triggered the immune system. Like other cases of AIH, no other confounding risk factors were identified. It is important to consider that the COVID-19 vaccine could be the inciting factor for Type 1 AIP.

Disclosures:
Erica C. Becker, MD, MPH1, Osama Siddique, MD2, Dinesh Kapur, MD3, Krishna Patel, MD2, Vaibhav Mehendiratta, MD2. B0031 - Type 1 Autoimmune Pancreatitis Unmasked by COVID-19 Vaccine, ACG 2022 Annual Scientific Meeting Abstracts. Charlotte, NC: American College of Gastroenterology.
1University of Connecticut Health Center, Farmington, CT; 2Hartford Hospital, Hartford, CT; 3William W. Backus Hospital, Norwich, CT
Introduction: Type 1 AIP is a rare form of idiopathic chronic pancreatitis associated with IgG4-related systemic disease. It predominantly affects male adults and has multi-organ extra-pancreatic manifestations. It is much less associated with inflammatory bowel disease compared to type 2 AIP, which is reported to have a least a 15% predilection for ulcerative colitis. To date there are no reports of vaccine-induced type 1 AIP. We present a case of newly diagnosed type 1 AIP in a patient one month after receiving COVID-19 vaccination.
Case Description/Methods: 54-year-old male with history of UC presented with decreased appetite, abdominal pain, fatigue, and 25 pound unintentional weight loss. He had received the second dose of Pfizer/BioNTech COVID-19 mRNA vaccine about 1 month prior. Laboratory tests were significant for hypovolemia, hyperglycemia, and elevated liver enzymes. IgG4 level was 287.0 mg/dL. CT scan showed distended pancreatic parenchyma with peri-pancreatic stranding with segments of biliary ductal dilation. MRCP showed dilated bile ducts to the level of the head of the pancreas where there was an abrupt truncation of the extrahepatic common duct. The pancreas was diffusely enlarged with some irregular beading and narrowing of the main pancreatic duct. On ERCP the lower third of the main bile duct contained a single severe stenosis 20-25 mm in length and a biliary stent was placed. EUS was performed and pancreatic parenchyma was diffusely abnormal with lobularity, generalized hypoechoic gland, hyperechoic foci without shadowing. The pancreatic duct was irregular in contour. Immunohistochemical tests showed IgG and IgG4 positive plasma cells. The patient was started on a three week prednisone taper. On 9 month follow-up clinical symptoms, laboratory work and repeat imaging showed improvement.
Discussion: Both vaccine-induced acute hepatitis and pancreatitis have been reported occurring 2-3 days after receiving Pfizer/BioNTech COVID-19 mRNA vaccine. Our case suggests a temporal association between COVID-19 vaccination and AIP although a cause effect relation cannot be definitely established. Our patient was the appropriate age and had ulcerative colitis; thus, the correct demographic for developing AIP. He may have been genetically predisposed and the vaccination triggered the immune system. Like other cases of AIH, no other confounding risk factors were identified. It is important to consider that the COVID-19 vaccine could be the inciting factor for Type 1 AIP.

Figure: Medium Power (10x) Immunohistochemical positivity for IgG on plasma cells.
Disclosures:
Erica Becker indicated no relevant financial relationships.
Osama Siddique indicated no relevant financial relationships.
Dinesh Kapur indicated no relevant financial relationships.
Krishna Patel indicated no relevant financial relationships.
Vaibhav Mehendiratta indicated no relevant financial relationships.
Erica C. Becker, MD, MPH1, Osama Siddique, MD2, Dinesh Kapur, MD3, Krishna Patel, MD2, Vaibhav Mehendiratta, MD2. B0031 - Type 1 Autoimmune Pancreatitis Unmasked by COVID-19 Vaccine, ACG 2022 Annual Scientific Meeting Abstracts. Charlotte, NC: American College of Gastroenterology.
