Back

Poster Session B - Monday Morning
Category: IBD
B0358 - Efficacy of the Melanocortin Receptor Agonist PL8177 as a Potential Therapy for Gastrointestinal (GI) Inflammatory Diseases
Monday, October 24, 2022
10:00 AM – 12:00 PM ET
Location: Crown Ballroom

Has Audio

John Dodd, PhD
Palatin Technologies, Inc.
Cranbury, NJ
Presenting Author(s)
John Dodd, PhD1, Robert Jordan, 1, Barry Koplowitz, MS2, Luana Pesco Koplowitz, MD2, Carl Spana, PhD1
1Palatin Technologies, Inc., Cranbury, NJ; 2DUCK FLATS Pharma, Flemington, NJ
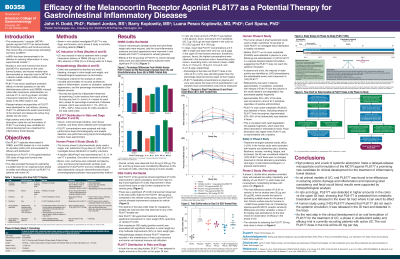
Introduction: Melanocortin-1 receptors (MC1Rs), found in many tissues including the colon, promote the resolution of inflammation. High potency and no detected systemic absorption make the MC1R agonist PL8177 a promising candidate for oral gut restricted ulcerative colitis (UC) treatment.
Methods: Oral PL8177 capsules were tested in 2 rat UC models and evaluated for distribution, in vivo, in rats, dogs, and humans. UC was induced in rats by dinitrobenzene sulfonic acid (DNBS) or dextran sulfate sodium (DSS). BID doses of 10, 20, 50, 100 or 200 µg PL8177 were compared with vehicle control (placebo) in DNBS and DSS rats. Colitis was assessed by diarrhea and rectal bleeding, and by changes in colon length and weight, and histopathological assessment on termination. Distribution of PL8177 within the GI tract after a single PL8177 dose was investigated in rats and dogs. A phase 0 study using a single oral microdose (70 µg) of [14C]-labeled PL8177 investigated the release of PL8177 in the colon of healthy men. A phase 2 double-blind, placebo-controlled, study will evaluate the safety, tolerability and efficacy of oral PL8177 in adults with UC.
Results: Rats treated with 10-200 µg oral PL8177 demonstrated lower macroscopic colon damage scores and improvement in colon weight, stool consistency, and fecal occult blood vs the vehicle. Histopathology analysis showed PL8177 treatment resulted in the maintenance of intact colon structure and barrier, and reduced immune cell infiltration. In rats and dogs, PL8177 was detected at higher amounts in the colon vs upper GI tract. [14C]-PL8177 and the main metabolite were not detected in human plasma and urine, suggesting that the parent drug [14C]PL8177 was released from the polymer formulation and metabolized within the GI tract. The most efficacious doses (P< 0.05 vs vehicle) in the rat studies were 20 and 50 µg, which provide the basis for estimating suitable doses for the phase 2 trial. Colonic surface area for humans is ~3,000 greater than rat. Considering species-specific MC1R receptor sensitivity differences and other variables, such as GI transit time, pH, and proteolytic enzyme activity, a dose of 20 mg/day was estimated to be the best choice for observation of efficacy in the phase 2 trial.
Discussion: Collectively, these findings support further development of PL8177 as a treatment option for GI inflammatory diseases in humans. This will be further examined in the phase 2 trial in humans with UC.
Disclosures:
John Dodd, PhD1, Robert Jordan, 1, Barry Koplowitz, MS2, Luana Pesco Koplowitz, MD2, Carl Spana, PhD1. B0358 - Efficacy of the Melanocortin Receptor Agonist PL8177 as a Potential Therapy for Gastrointestinal (GI) Inflammatory Diseases, ACG 2022 Annual Scientific Meeting Abstracts. Charlotte, NC: American College of Gastroenterology.
1Palatin Technologies, Inc., Cranbury, NJ; 2DUCK FLATS Pharma, Flemington, NJ
Introduction: Melanocortin-1 receptors (MC1Rs), found in many tissues including the colon, promote the resolution of inflammation. High potency and no detected systemic absorption make the MC1R agonist PL8177 a promising candidate for oral gut restricted ulcerative colitis (UC) treatment.
Methods: Oral PL8177 capsules were tested in 2 rat UC models and evaluated for distribution, in vivo, in rats, dogs, and humans. UC was induced in rats by dinitrobenzene sulfonic acid (DNBS) or dextran sulfate sodium (DSS). BID doses of 10, 20, 50, 100 or 200 µg PL8177 were compared with vehicle control (placebo) in DNBS and DSS rats. Colitis was assessed by diarrhea and rectal bleeding, and by changes in colon length and weight, and histopathological assessment on termination. Distribution of PL8177 within the GI tract after a single PL8177 dose was investigated in rats and dogs. A phase 0 study using a single oral microdose (70 µg) of [14C]-labeled PL8177 investigated the release of PL8177 in the colon of healthy men. A phase 2 double-blind, placebo-controlled, study will evaluate the safety, tolerability and efficacy of oral PL8177 in adults with UC.
Results: Rats treated with 10-200 µg oral PL8177 demonstrated lower macroscopic colon damage scores and improvement in colon weight, stool consistency, and fecal occult blood vs the vehicle. Histopathology analysis showed PL8177 treatment resulted in the maintenance of intact colon structure and barrier, and reduced immune cell infiltration. In rats and dogs, PL8177 was detected at higher amounts in the colon vs upper GI tract. [14C]-PL8177 and the main metabolite were not detected in human plasma and urine, suggesting that the parent drug [14C]PL8177 was released from the polymer formulation and metabolized within the GI tract. The most efficacious doses (P< 0.05 vs vehicle) in the rat studies were 20 and 50 µg, which provide the basis for estimating suitable doses for the phase 2 trial. Colonic surface area for humans is ~3,000 greater than rat. Considering species-specific MC1R receptor sensitivity differences and other variables, such as GI transit time, pH, and proteolytic enzyme activity, a dose of 20 mg/day was estimated to be the best choice for observation of efficacy in the phase 2 trial.
Discussion: Collectively, these findings support further development of PL8177 as a treatment option for GI inflammatory diseases in humans. This will be further examined in the phase 2 trial in humans with UC.
Disclosures:
John Dodd: Palatin Technologies, Inc. – Employee.
Robert Jordan: Palatin Technologies, Inc. – Employee.
Barry Koplowitz: Palatin Technologies, Inc. – Consultant.
Luana Pesco Koplowitz: Palatin Technologies, Inc. – Consultant.
Carl Spana: Palatin Technologies, Inc. – Employee.
John Dodd, PhD1, Robert Jordan, 1, Barry Koplowitz, MS2, Luana Pesco Koplowitz, MD2, Carl Spana, PhD1. B0358 - Efficacy of the Melanocortin Receptor Agonist PL8177 as a Potential Therapy for Gastrointestinal (GI) Inflammatory Diseases, ACG 2022 Annual Scientific Meeting Abstracts. Charlotte, NC: American College of Gastroenterology.

