Back

Poster Session B - Monday Morning
Category: IBD
B0363 - Serum Ustekinumab Concentrations Are Associated With Improved Outcomes With the Magnetic Resonance Index of Activity for Crohn's Disease
Monday, October 24, 2022
10:00 AM – 12:00 PM ET
Location: Crown Ballroom

Has Audio

Kaylie Chen, BS
Weill Cornell Medicine
New York, NY
Presenting Author(s)
Award: Presidential Poster Award
Kaylie Chen, BS1, Johnson Chen, MD1, Emily Smith, MD1, Prerna Mahtani, MS1, Randy Longman, MD, PhD1, Dana Lukin, MD, PhD1, Ellen J. Scherl, MD2, Juliette Gerber, BS1, Robert Battat, MD1
1Weill Cornell Medicine, New York, NY; 2Weill Cornell Medicine, New York Presbyterian Hospital, New York, NY
Introduction: Controversy exists for ustekinumab concentrations needed in Crohn’s disease (CD). No data exist comparing ustekinumab concentrations and validated radiologic outcomes. We characterized these relationships and clarified concentrations needed.
Methods: CD patients on maintenance ( > 16 weeks) ustekinumab with both ustekinumab concentrations and simplified magnetic resonance index of activity (sMaRIA) scoring were included. Ustekinumab concentrations were compared between those with and without (1) radiologic remission (sMaRIA < 2), (2) severe radiologic inflammation (sMaRIA < 3) and (3) fecal calprotectin (FCP) biomarker remission (FCP < 50 μg/g). Area under the receiver-operating characteristic (AUROC) curve determined optimal ustekinumab concentrations. Outcomes were compared between patients above and below identified ustekinumab thresholds.
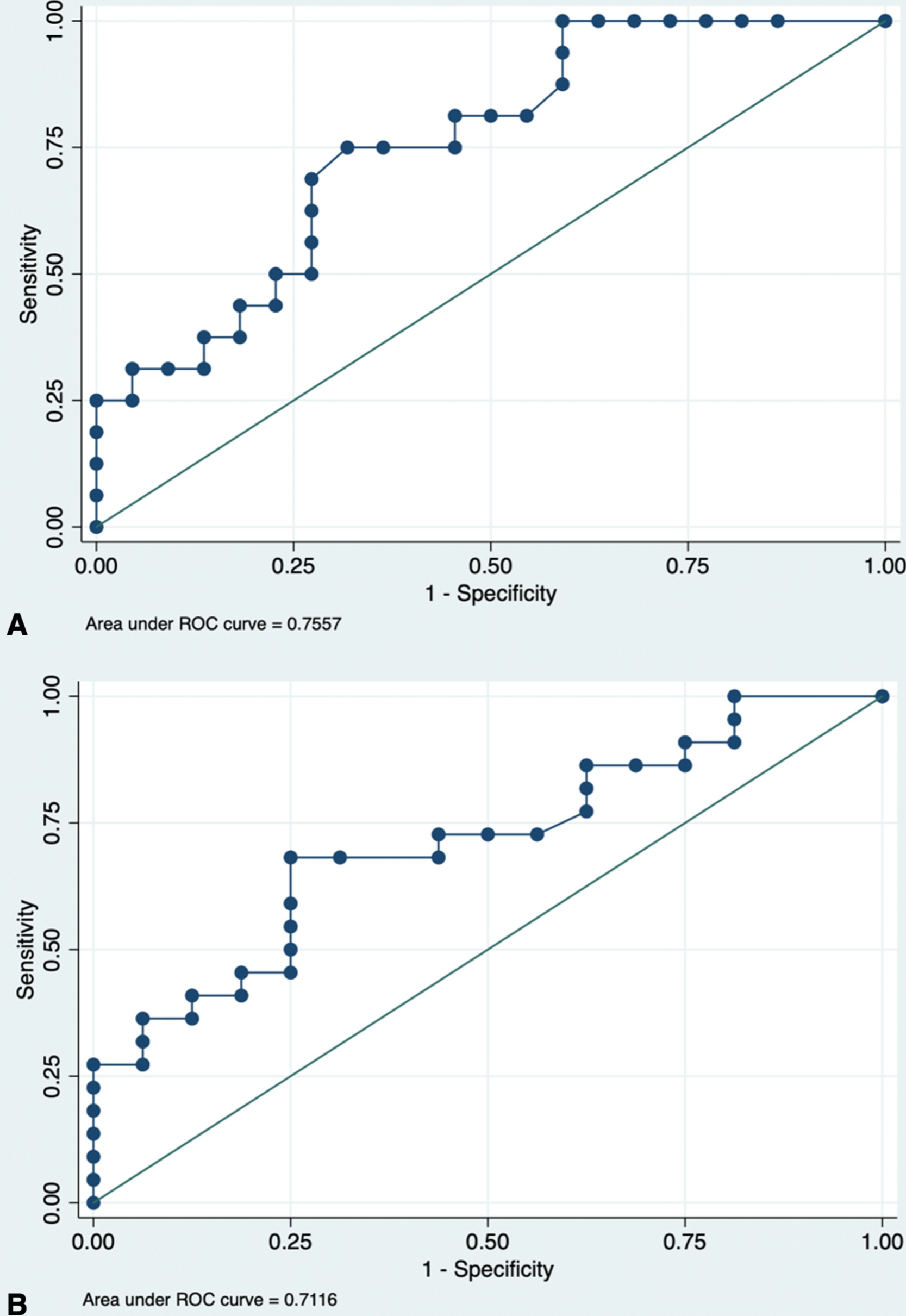
Results: Thirty-eight paired ustekinumab concentrations and MRE were included. Ustekinumab concentrations were higher with radiologic remission (11.4 μg/mL vs. 6.4 μg/mL, P=.005) and had good diagnostic accuracy for radiologic remission (AUROC 0.76, 95% CI 0.60 – 0.91) and for absence of severe inflammation (AUROC 0.71, 95% CI 0.55 – 0.88, optimal concentration 8.4 μg/mL). With ustekinumab ≥ 8.4 μg/mL, higher proportions had radiologic remission (63.2% vs. 21.1%, P=.01) and absence of severe inflammation (78.9% vs. 36.8%, P=.01) compared to patients with lower concentrations. Ustekinumab concentrations had good diagnostic accuracy (AUROC 0.73, 95% CI 0.52 – 0.94) for FCP biomarker remission (optimal concentration: 6.1 μg/mL). Patients with ustekinumab concentrations ≥ 6.1 μg/mL had higher proportions with biomarker remission (72.2% vs. 12.5% P < .01) compared to those with lower concentrations.
Discussion: Ustekinumab concentrations are associated with radiologic and biomarker outcomes in CD. These data validate the need for higher ustekinumab concentrations.
Disclosures:
Kaylie Chen, BS1, Johnson Chen, MD1, Emily Smith, MD1, Prerna Mahtani, MS1, Randy Longman, MD, PhD1, Dana Lukin, MD, PhD1, Ellen J. Scherl, MD2, Juliette Gerber, BS1, Robert Battat, MD1. B0363 - Serum Ustekinumab Concentrations Are Associated With Improved Outcomes With the Magnetic Resonance Index of Activity for Crohn's Disease, ACG 2022 Annual Scientific Meeting Abstracts. Charlotte, NC: American College of Gastroenterology.
Kaylie Chen, BS1, Johnson Chen, MD1, Emily Smith, MD1, Prerna Mahtani, MS1, Randy Longman, MD, PhD1, Dana Lukin, MD, PhD1, Ellen J. Scherl, MD2, Juliette Gerber, BS1, Robert Battat, MD1
1Weill Cornell Medicine, New York, NY; 2Weill Cornell Medicine, New York Presbyterian Hospital, New York, NY
Introduction: Controversy exists for ustekinumab concentrations needed in Crohn’s disease (CD). No data exist comparing ustekinumab concentrations and validated radiologic outcomes. We characterized these relationships and clarified concentrations needed.
Methods: CD patients on maintenance ( > 16 weeks) ustekinumab with both ustekinumab concentrations and simplified magnetic resonance index of activity (sMaRIA) scoring were included. Ustekinumab concentrations were compared between those with and without (1) radiologic remission (sMaRIA < 2), (2) severe radiologic inflammation (sMaRIA < 3) and (3) fecal calprotectin (FCP) biomarker remission (FCP < 50 μg/g). Area under the receiver-operating characteristic (AUROC) curve determined optimal ustekinumab concentrations. Outcomes were compared between patients above and below identified ustekinumab thresholds.
Results: Thirty-eight paired ustekinumab concentrations and MRE were included. Ustekinumab concentrations were higher with radiologic remission (11.4 μg/mL vs. 6.4 μg/mL, P=.005) and had good diagnostic accuracy for radiologic remission (AUROC 0.76, 95% CI 0.60 – 0.91) and for absence of severe inflammation (AUROC 0.71, 95% CI 0.55 – 0.88, optimal concentration 8.4 μg/mL). With ustekinumab ≥ 8.4 μg/mL, higher proportions had radiologic remission (63.2% vs. 21.1%, P=.01) and absence of severe inflammation (78.9% vs. 36.8%, P=.01) compared to patients with lower concentrations. Ustekinumab concentrations had good diagnostic accuracy (AUROC 0.73, 95% CI 0.52 – 0.94) for FCP biomarker remission (optimal concentration: 6.1 μg/mL). Patients with ustekinumab concentrations ≥ 6.1 μg/mL had higher proportions with biomarker remission (72.2% vs. 12.5% P < .01) compared to those with lower concentrations.
Discussion: Ustekinumab concentrations are associated with radiologic and biomarker outcomes in CD. These data validate the need for higher ustekinumab concentrations.

Figure: Figure 1. (A) Receiver-operating characteristic curve analysis for sMaRIA score of < 2, absence of any inflammation, based on ustekinumab trough concentrations. The optimal concentration is 8.4 μg/mL as indicated by the best receiver-operating characteristic curve (area under curve, 0.76; sensitivity, 75%; specificity 68%). (B) Receiver-operating characteristic curve analysis for sMaRIA score of < 3, absence of severe inflammation, based on ustekinumab concentrations. The optimal serum concentration is 8.4 μg/mL as indicated by the best receiver-operating characteristic curve (area under curve, 0.71).
Disclosures:
Kaylie Chen indicated no relevant financial relationships.
Johnson Chen indicated no relevant financial relationships.
Emily Smith indicated no relevant financial relationships.
Prerna Mahtani indicated no relevant financial relationships.
Randy Longman: Ancilia Biosciences – Consultant. Enzymetrics – Consultant. Pfizer – Consultant.
Dana Lukin: AbbVie – Consultant, Grant/Research Support. Boehringer Ingelheim – Consultant. Bristol Myers Squibb – Consultant. Eli Lilly – Consultant. Janssen – Consultant, Grant/Research Support. Palatin Technologies – Consultant. Pfizer – Consultant. Takeda – Grant/Research Support.
Ellen Scherl: AbbVie – Consultant, Grant/Research Support. AstraZeneca – Grant/Research Support. Bristol Myers Squibb – Consultant. Celgene – Grant/Research Support. Crohn’s and Colitis Foundation – Consultant, Grant/Research Support. Entera Health – Consultant. Evidera – Consultant. Genentech – Grant/Research Support. GI Health Foundation – Consultant, Speakers Bureau. Gilead – Stock Options. Janssen Research & Development, LLC – Consultant, Grant/Research Support. Johns Hopkins University – Grant/Research Support. National Institute of Diabetes and Digestive and Kidney – Grant/Research Support. National Institute of Health – Grant/Research Support. New York Crohn’s Foundation – Grant/Research Support. Pfizer – Grant/Research Support. Protagonist – Consultant. Seres – Consultant, Grant/Research Support. Takeda – Consultant. UCB – Grant/Research Support.
Juliette Gerber indicated no relevant financial relationships.
Robert Battat indicated no relevant financial relationships.
Kaylie Chen, BS1, Johnson Chen, MD1, Emily Smith, MD1, Prerna Mahtani, MS1, Randy Longman, MD, PhD1, Dana Lukin, MD, PhD1, Ellen J. Scherl, MD2, Juliette Gerber, BS1, Robert Battat, MD1. B0363 - Serum Ustekinumab Concentrations Are Associated With Improved Outcomes With the Magnetic Resonance Index of Activity for Crohn's Disease, ACG 2022 Annual Scientific Meeting Abstracts. Charlotte, NC: American College of Gastroenterology.

