Poster Session A - Sunday Afternoon
Category: Colorectal Cancer Prevention
A0178 - Colonoscopy Quality Metrics After a Multi-Target Stool DNA or Fecal Immunochemical Test: A Systematic Review and Meta-Analysis

Rami Musallam, MD
St. Vincent Charity Medical Center
Cleveland, OH
Presenting Author(s)
1St. Vincent Charity Medical Center, Cleveland, OH; 2University of Toledo, Toledo, OH; 3CHI Health Creighton School of Medicine, Omaha, NE; 4University of Utah School of Medicine, Salt Lake City, UT; 5Cleveland Clinic Foundation, Cleveland, OH
Introduction: Colorectal cancer (CRC) accounts for approximately 50,000 deaths or 14 deaths per 100,000 people yearly in the United States. Multi-target stool DNA (mt-sDNA) and fecal immunochemical test (FIT) are validated CRC screening strategies in average-risk asymptomatic individuals. This study aims to evaluate the colonoscopy quality metrics following a positive mt-sDNA test, FIT.
Methods: We performed a comprehensive search in the databases of PubMed/MEDLINE, Embase, and the Cochrane Central Register of Controlled Trials from inception through May 2022. Meta-analysis was performed by standard methodology using the random-effects model and heterogeneity was assessed using the I2% statistics. Outcomes of interest were adenoma detection rate (ADR), colorectal cancer detection rate (CRCDR), withdrawal time (WT), and cecal intubation rate (CIR).
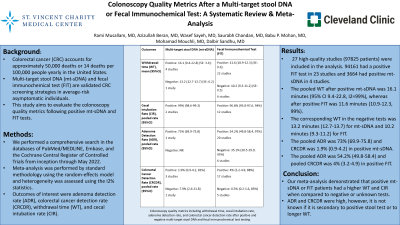
Results: 27 high-quality studies (97825 patients) were included in the analysis. 94161 had a positive FIT test in 23 studies and 3664 had positive mt-sDNA in 4 studies. The pooled WT after positive mt-sDNA was 16.1 minutes (95% CI 9.4-22.8, I2=99%), whereas after positive FIT was 11.6 minutes (10.9-12.3, 99%). The corresponding WT in the negative tests was 13.2 minutes (12.7-13.7) for mt-sDNA and 10.2 minutes (9.3-11.2) for FIT. The pooled ADR was 73% (69.9-75.8) and CRCDR was 1.9% (0.9-4.2) in mt-sDNA positive. The pooled ADR was 54.2% (49.8-58.4) and pooled CRCDR was 4% (3.2-4.9) in FIT positive. Pooled CIR were excellent: 99% (98.6-99.3) in mt-sDNA positive, and 96.8% (95.8-97.6) in FIT positive. Pooled rates are summarized in Table 1.
Discussion: Our meta-analysis demonstrated that positive mt-sDNA or FIT patients had a higher WT and CIR when compared to negative or unknown tests. ADR and CRCDR were high, however, it is not known if it is secondary to positive stool test or to longer WT. Future studies are needed to validate our findings and determine the cost-effectiveness of these screening tests.
Outcomes | Multi-target stool DNA (mt-sDNA) | Fecal Immunochemical Test (FIT) |
Withdrawal time (WT), mean (95%CI) | Positive: 16.1 (9.4-22.8) (SE: 3.4); 4 studies
Negative: 13.2 (12.7-13.7) (SE: 0.2) 1 study | Positive: 11.6 (10.9-12.3) (SE: 0.3); 22 studies
Negative: 10.2 (9.3-11.2) (SE: 0.5) 6 studies |
Cecal intubation Rate (CIR), pooled rate (95%CI) | Positive: 99% (98.6-99.3) 2 studies | Positive: 96.8% (95.8-97.6, 94%) 12 studies |
Adenoma Detection Rate (ADR), pooled rate (95%CI) | Positive: 73% (69.9-75.8) 1 study
Negative: NR | Positive: 54.2% (49.8-58.4, 97%) 20 studies
Negative: 35.1% (30.5-39.9, 95%) 6 studies |
Colorectal Cancer Detection Rate (CRCDR), pooled rate (95%CI) | Positive: 1.9% (0.9-4.2, 81%) 3 studies
Negative: 7.9% (2.6-21.8) 1 study
| Positive: 4% (3.2-4.9, 88%) 17 studies
Negative: 0.5% (0.2-1.6, 85%) 5 studies |
Abbreviations: NR: not reported, SE: standard error, CI: confidence interval
Disclosures:
Rami Musallam, MD1, Azizullah Beran, MD2, Wasef Sayeh, MD2, Saurabh Chandan, MD3, Babu P. Mohan, MD, MS4, Mohamad Mouchli, MD5, Dalbir Sandhu, MD5. A0178 - Colonoscopy Quality Metrics After a Multi-Target Stool DNA or Fecal Immunochemical Test: A Systematic Review and Meta-Analysis, ACG 2022 Annual Scientific Meeting Abstracts. Charlotte, NC: American College of Gastroenterology.
