Back
Poster Session B - Monday Morning
Category: IBD
B0370 - Presence of Risk Factors Associated With Colectomy Among Patients With Colectomy in the Tofacitinib OCTAVE Ulcerative Colitis Clinical Program
Monday, October 24, 2022
10:00 AM – 12:00 PM ET
Location: Crown Ballroom


David T. Rubin, MD, FACG
University of Chicago Medicine Inflammatory Bowel Disease Center
Chicago, IL
Presenting Author(s)
David T. Rubin, MD, FACG1, Leonardo Salese, MD2, Paulo G. Kotze, MD, MSC, PhD3, John C. Woolcott, PhD2, Chinyu Su, MD2, Rajiv Mundayat, MSc4, Jerome Paulissen, MS4, Joana Torres, MD, PhD5, Millie Long, MD, MPH, FACG6
1University of Chicago Medicine Inflammatory Bowel Disease Center, Chicago, IL; 2Pfizer Inc, Collegeville, PA; 3Catholic University of Paraná, Curitiba, Parana, Brazil; 4Pfizer Inc, New York, NY; 5Hospital Beatriz Ângelo, Loures, Portugal and Hospital da Luz, Lisbon, Lisboa, Portugal; 6UNC Chapel Hill, Chapel Hill, NC
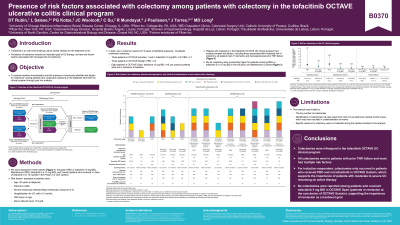
Introduction: Tofacitinib is an oral small molecule JAK inhibitor for the treatment of UC. Avoidance of colectomy remains an important goal of UC therapy and there are known factors associated with increased colectomy risk.1
Methods: This post hoc analysis assessed baseline characteristics and presence of risk factors for colectomy among patients (pts) who underwent colectomy in the tofacitinib OCTAVE clinical program (NCT01465763;NCT01458951;NCT01458574;NCT01470612). Incidence rates (IRs; unique pts with events/100 pt-years [PY] of exposure) were assessed in 3 cohorts: Induction (placebo [PBO] or tofacitinib 10 mg twice daily [BID]), Maintenance (PBO, tofacitinib 5 or 10 mg BID), and Overall (pts who received ≥ 1 dose of tofacitinib 5 or 10 mg BID in Phase 3 or open-label, long-term extension [OLE] studies). Risk factors assessed were: age < 40 years at diagnosis, extensive colitis, severe endoscopic disease (Mayo endoscopic subscore of 3), hospitalization for colitis within 12 months, C-reactive protein > 3 mg/L, and serum albumin < 3.5 g/dL.1
Results: In total, 14 pts underwent colectomy: 3/1,139 in the induction studies (tofacitinib 10 mg BID: n = 2; PBO: n = 1), 3/593 in the maintenance study (PBO: n = 3), and 8/944 in the OLE study (tofacitinib 10 mg BID: n = 8; per protocol pts were not in remission at baseline). IRs per 100 PY (95% CI; exposure) were: Induction: PBO, 2.47 (0.06, 13.74; 40.55 PY) and tofacitinib 10 mg BID, 1.26 (0.15, 4.55; 158.72 PY); Maintenance: PBO, 2.90 (0.60, 8.47; 103.48 PY), tofacitinib 5 mg BID, 0.00 (0.00, 2.48; 148.77 PY), and tofacitinib 10 mg BID, 0.00 (0.00, 2.35; 157.31 PY); Overall: tofacitinib all, 0.34 (0.16, 0.63; 2915.95 PY). Baseline characteristics of pts who underwent colectomy, including treatment duration and risk factors for colectomy, are summarized (Table); all had ≥ 1 risk factor and prior tumor necrosis factor inhibitor (TNFi) exposure.
Discussion: Colectomies were infrequent in the OCTAVE clinical program; all were in pts with prior TNFi exposure and most pts had multiple risk factors. For induction responders, the fact that colectomies only occurred in pts who received PBO and not tofacitinib in the maintenance study supports the importance of continued active therapy in pts with moderate to severe UC. This analysis was limited by the low number of colectomies and potential underestimation due to case report form identification without adjudication.
Reference
Disclosures:
David T. Rubin, MD, FACG1, Leonardo Salese, MD2, Paulo G. Kotze, MD, MSC, PhD3, John C. Woolcott, PhD2, Chinyu Su, MD2, Rajiv Mundayat, MSc4, Jerome Paulissen, MS4, Joana Torres, MD, PhD5, Millie Long, MD, MPH, FACG6. B0370 - Presence of Risk Factors Associated With Colectomy Among Patients With Colectomy in the Tofacitinib OCTAVE Ulcerative Colitis Clinical Program, ACG 2022 Annual Scientific Meeting Abstracts. Charlotte, NC: American College of Gastroenterology.
1University of Chicago Medicine Inflammatory Bowel Disease Center, Chicago, IL; 2Pfizer Inc, Collegeville, PA; 3Catholic University of Paraná, Curitiba, Parana, Brazil; 4Pfizer Inc, New York, NY; 5Hospital Beatriz Ângelo, Loures, Portugal and Hospital da Luz, Lisbon, Lisboa, Portugal; 6UNC Chapel Hill, Chapel Hill, NC
Introduction: Tofacitinib is an oral small molecule JAK inhibitor for the treatment of UC. Avoidance of colectomy remains an important goal of UC therapy and there are known factors associated with increased colectomy risk.1
Methods: This post hoc analysis assessed baseline characteristics and presence of risk factors for colectomy among patients (pts) who underwent colectomy in the tofacitinib OCTAVE clinical program (NCT01465763;NCT01458951;NCT01458574;NCT01470612). Incidence rates (IRs; unique pts with events/100 pt-years [PY] of exposure) were assessed in 3 cohorts: Induction (placebo [PBO] or tofacitinib 10 mg twice daily [BID]), Maintenance (PBO, tofacitinib 5 or 10 mg BID), and Overall (pts who received ≥ 1 dose of tofacitinib 5 or 10 mg BID in Phase 3 or open-label, long-term extension [OLE] studies). Risk factors assessed were: age < 40 years at diagnosis, extensive colitis, severe endoscopic disease (Mayo endoscopic subscore of 3), hospitalization for colitis within 12 months, C-reactive protein > 3 mg/L, and serum albumin < 3.5 g/dL.1
Results: In total, 14 pts underwent colectomy: 3/1,139 in the induction studies (tofacitinib 10 mg BID: n = 2; PBO: n = 1), 3/593 in the maintenance study (PBO: n = 3), and 8/944 in the OLE study (tofacitinib 10 mg BID: n = 8; per protocol pts were not in remission at baseline). IRs per 100 PY (95% CI; exposure) were: Induction: PBO, 2.47 (0.06, 13.74; 40.55 PY) and tofacitinib 10 mg BID, 1.26 (0.15, 4.55; 158.72 PY); Maintenance: PBO, 2.90 (0.60, 8.47; 103.48 PY), tofacitinib 5 mg BID, 0.00 (0.00, 2.48; 148.77 PY), and tofacitinib 10 mg BID, 0.00 (0.00, 2.35; 157.31 PY); Overall: tofacitinib all, 0.34 (0.16, 0.63; 2915.95 PY). Baseline characteristics of pts who underwent colectomy, including treatment duration and risk factors for colectomy, are summarized (Table); all had ≥ 1 risk factor and prior tumor necrosis factor inhibitor (TNFi) exposure.
Discussion: Colectomies were infrequent in the OCTAVE clinical program; all were in pts with prior TNFi exposure and most pts had multiple risk factors. For induction responders, the fact that colectomies only occurred in pts who received PBO and not tofacitinib in the maintenance study supports the importance of continued active therapy in pts with moderate to severe UC. This analysis was limited by the low number of colectomies and potential underestimation due to case report form identification without adjudication.
Reference
- Rubin DT et al. Am J Gastroenterol 2019;114:384–413
| Patients with UC in OCTAVE Induction 1&2 (8 weeks [NCT01465763; NCT01458951]), OCTAVE Sustain (52 weeks [NCT01458574]) and OCTAVE Open (OLE study [NCT01470612]) were evaluated. All patients had prior TNFi exposure aData were taken from baseline of the respective studies in which the colectomy occurred bData were taken from baseline of OCTAVE Induction 1&2 cRisk factors for colectomy included: age < 40 years at diagnosis, extensive colitis, severe endoscopic disease (MES of 3), hospitalization for colitis within 12 months, CRP > 3 mg/L, and serum albumin < 3.5 g/dL BID, twice daily; CRP, C-reactive protein; MES, Mayo endoscopic subscore; OLE, open-label, long-term extension; TNFi, tumor necrosis factor inhibitor; UC, ulcerative colitis | |||||||||
| Table. Baseline Demographics and Clinical Characteristics of Patients Who Underwent Colectomy in the OCTAVE Clinical Program | |||||||||
|---|---|---|---|---|---|---|---|---|---|
| Treatment group at time of colectomy | Sex, Age at diagnosis (years), Disease duration (years)a | Disease extent | MES at baseline | Hospitalization for colitis within 12 months | Baseline | Baseline albumin (g/dL) | Days of treatment prior to colectomy | Total Mayo score at last visit prior to colectomy | Risk factors presentb,c |
| OCTAVE Induction 1&2 | |||||||||
| Tofacitinib 10 mg BID | Male, 26, ≥ 6 | Left-sided colitis | 3 | No | 53.53 | 4.0 | 21 | 11 | 3 |
| Tofacitinib 10 mg BID | Male, 41, < 6 | Left-sided colitis | 3 | No | 1.90 | 4.3 | 38 | 11 | 1 |
| Placebo | Female, 14, < 6 | Extensive/pancolitis | 2 | No | 4.36 | 3.6 | 58 | 9 | 3 |
| OCTAVE Sustain | |||||||||
| Placebo | Female, 52, ≥ 6 | Extensive/pancolitis | 3 | Yes | 4.65 | 4.0 | 126 | 6 | 4 |
| Placebo | Female, 67, < 6 | Left-sided colitis | 3 | Yes | 19.59 | 3.9 | 143 | 10 | 3 |
| Placebo | Female, 23, ≥ 6 | Left-sided colitis | 3 | No | 1.08 | 4.5 | 63 | 4 | 2 |
| OCTAVE Open | |||||||||
| Tofacitinib 10 mg BID | Female, 35, ≥ 6 | Extensive/pancolitis | 3 | No | 13.42 | 4.1 | 62 | 12 | 4 |
| Tofacitinib 10 mg BID | Female, 22, ≥ 6 | Extensive/pancolitis | 3 | No | 4.02 | 4.2 | 132 | 6 | 4 |
| Tofacitinib 10 mg BID | Male, 44, < 6 | Proctosigmoiditis | 3 | No | 4.50 | 3.7 | 44 | 11 | 2 |
| Tofacitinib 10 mg BID | Female, 20, ≥ 6 | Extensive/pancolitis | 3 | No | 36.80 | 4.2 | 92 | 4 | 4 |
| Tofacitinib 10 mg BID | Male, 31, < 6 | Extensive/pancolitis | 3 | Yes | 1.71 | 4.3 | 56 | 8 | 4 |
| Tofacitinib 10 mg BID | Male, 45, ≥ 6 | Extensive/pancolitis | 3 | Yes | 40.53 | 3.2 | 375 | 11 | 5 |
| Tofacitinib 10 mg BID | Female, 31, ≥ 6 | Left-sided colitis | 3 | Yes | 4.34 | 4.1 | 47 | 11 | 4 |
| Tofacitinib 10 mg BID | Female, 29, < 6 | Extensive/pancolitis | 3 | Yes | 7.50 | 4.0 | 174 | 6 | 5 |
Disclosures:
David Rubin: AbbVie – Consultant. Alimentiv Inc. – Consultant. AltruBio – Consultant. Arena Pharmaceuticals – Consultant. Aslan Pharmaceuticals – Consultant. Athos Therapeutics – Consultant. Bellatrix Pharmaceuticals – Consultant. Boehringer Ingelheim, Ltd. – Consultant. Bristol Myers Squibb – Consultant. Celgene Corp/Syneos – Consultant. ClostraBio – Consultant. Connect BioPharma – Consultant. EcoR1 – Consultant. Genentech/Roche – Consultant. Gilead Sciences – Consultant. Ironwood Pharmaceuticals – Consultant. Iterative Scopes – Consultant. Janssen – Consultant. Kaleido Biosciences – Consultant. Lilly, Eli & Co – Consultant. Materia Prima – Consultant. Pfizer – Consultant. Prometheus Biosciences – Consultant. Reistone Biopharma – Consultant. Seres Therapeutics, Inc – Consultant. Takeda – Consultant, Grant/Research Support. Target RWE – Consultant. Techlab – Consultant. Trellus Health – Consultant.
Leonardo Salese: Janssen Pharmaceuticals – Employee. Pfizer Inc – Employee, Shareholder.
Paulo Kotze: AbbVie – Consultant, Speaker fees. Janssen Pharmaceuticals – Consultant, Speaker fees. Pfizer Inc – Consultant, Grant/Research Support, Speaker fees. Takeda – Consultant, Grant/Research Support, Speaker fees.
John Woolcott: Pfizer Inc – Employee, Stock Options.
Chinyu Su: Pfizer Inc – Employee, Stock Options.
Rajiv Mundayat: Pfizer Inc – Employee, Stock Options.
Jerome Paulissen: Pfizer Inc – Contractor. Syneos Health – Employee.
Joana Torres: AbbVie – Advisory Committee/Board Member, Grant/Research Support, Speaker fees. Arena – Advisory Committee/Board Member. Galapagos – Advisory Committee/Board Member, Lecture fees. Janssen Pharmaceuticals – Advisory Committee/Board Member, Grant/Research Support, Lecture fees. Pfizer Inc – Advisory Committee/Board Member.
Millie Long: AbbVie – Consultant. Bristol Myers Squibb – Consultant. Calibr – Consultant. Eli Lilly – Consultant. Genentech – Consultant. Janssen – Consultant. Pfizer – Consultant, Grant/Research Support. Prometheus – Consultant. Roche – Consultant. Salix – Consultant. Takeda – Consultant, Grant/Research Support. Target RWE – Consultant. Theravance – Consultant.
David T. Rubin, MD, FACG1, Leonardo Salese, MD2, Paulo G. Kotze, MD, MSC, PhD3, John C. Woolcott, PhD2, Chinyu Su, MD2, Rajiv Mundayat, MSc4, Jerome Paulissen, MS4, Joana Torres, MD, PhD5, Millie Long, MD, MPH, FACG6. B0370 - Presence of Risk Factors Associated With Colectomy Among Patients With Colectomy in the Tofacitinib OCTAVE Ulcerative Colitis Clinical Program, ACG 2022 Annual Scientific Meeting Abstracts. Charlotte, NC: American College of Gastroenterology.
