Back


Poster Session B - Monday Morning
Category: IBD
B0376 - Risankizumab Results in Improvements in Disease Activity Scores in Patients With Crohn’s Disease: Post-Hoc Analysis of the Phase 3 Induction and Maintenance Studies
Monday, October 24, 2022
10:00 AM – 12:00 PM ET
Location: Crown Ballroom

Has Audio
- EL
Edward V. Loftus, Jr., MD, FACG
Mayo Clinic College of Medicine and Science
Rochester, Minnesota
Presenting Author(s)
Edward V. Loftus, MD, FACG1, Marc Ferrante, MD, PhD2, Jean-Frederic Colombel, MD3, Kristina Kligys, PhD4, Stijn Van Haaren, MsC4, Alexandra Song, MD4, Ezequiel Neimark, MD4, Javier Zambrano, MD4, Xiaomei Liao, PhD4, Marla Dubinsky, MD5
1Mayo Clinic College of Medicine and Science, Rochester, MN; 2University Hospitals Leuven, Leuven, Brabant Wallon, Belgium; 3Icahn School of Medicine at Mount Sinai, New York, NY; 4AbbVie, Inc., North Chicago, IL; 5Icahn School of Medicine, Mount Sinai, NY
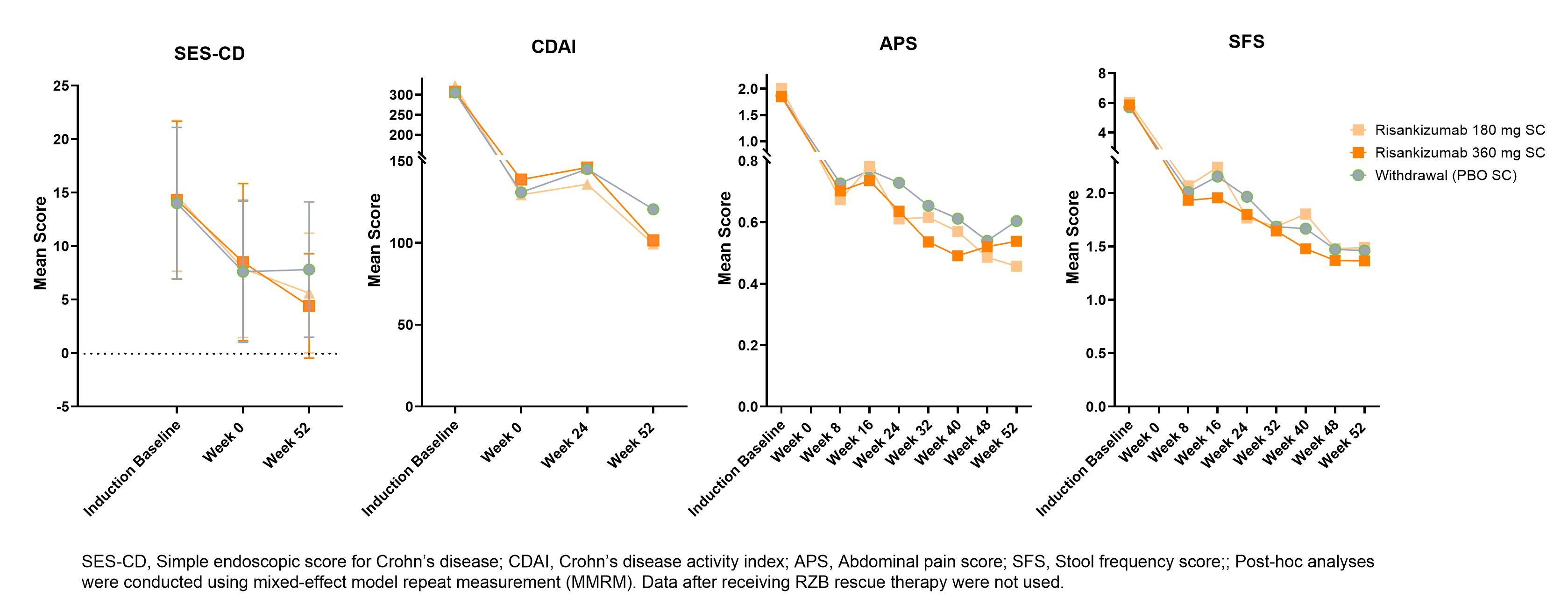
Introduction: The Phase 3 maintenance study (FORTIFY) was a re-randomized responder withdrawal study that demonstrated efficacy and safety of risankizumab (RZB), an anti-p19 IL-23 antibody, versus (vs) withdrawal/placebo (PBO) in patients with moderate to severe Crohn’s disease (CD) who responded to RZB induction therapy. In this post-hoc analysis, we evaluated disease activity, as measured by Simple Endoscopic Score for CD (SES-CD), Crohn's Disease Activity Index (CDAI), and patient reported symptoms (PROs) of liquid stool frequency (SF) and abdominal pain score (APS), in patients who received up to 52 weeks (wks) of RZB or PBO in FORTIFY.
Methods: Patients enrolled in FORTIFY achieved clinical response to IV RZB induction therapy in ADVANCE and MOTIVATE studies. Clinical response was defined as ≥30% decrease in daily SF and/or ≥30% decrease in average daily APS from induction baseline [BL])both not worse than induction BL. In FORTIFY, patients were dosed Q8W with 180 mg subcutaneous (SC) RZB, 360 mg SC RZB, or PBO. For patients who entered FORTIFY, mean SES-CD and CDAI values were calculated at induction BL and at FORTIFY Wks 0, 24 (CDAI only), and 52; mean SF and APS were calculated from patient diary data at BL of induction and at Wks 8, 16, 24, 32, 40, 48, and 52 of FORTIFY.
Results: At induction BL, mean SES-CD, CDAI, SF, and AP scores were similar between treatment groups. At FORTIFY Wk 0, disease activity scores improved from induction BL for all patients, consistent with an IV RZB induction responder population for FORTIFY. Over time, patients on RZB maintenance therapy demonstrated ongoing improvement in SES-CD and CDAI values, whereas mean SES-CD and CDAI values increased in the withdrawal/PBO group. A treatment effect was evident at Wk 52. For most timepoints throughout maintenance, patients receiving RZB showed numerically lower APS and SF (RZB 360 mg only) compared to patients in the withdrawal/PBO group.
Discussion: Across the studies, RZB therapy led to marked improvements in disease activity over time and continued to show benefit compared to withdrawal/PBO at Wk 52. The pronounced benefit of RZB over withdrawal/PBO at Wk 52 for SES-CD, an objective endoscopic endpoint, contrasts with a relative lack of differentiation at Wk 52 for the subjective SF and AP endpoints. This is likely explained by residual exposure given the long half-life and pharmacodynamic effect of RZB following induction therapy, leading to prolonged symptom improvement in patients receiving PBO in maintenance.
Disclosures:
Edward V. Loftus, MD, FACG1, Marc Ferrante, MD, PhD2, Jean-Frederic Colombel, MD3, Kristina Kligys, PhD4, Stijn Van Haaren, MsC4, Alexandra Song, MD4, Ezequiel Neimark, MD4, Javier Zambrano, MD4, Xiaomei Liao, PhD4, Marla Dubinsky, MD5. B0376 - Risankizumab Results in Improvements in Disease Activity Scores in Patients With Crohn’s Disease: Post-Hoc Analysis of the Phase 3 Induction and Maintenance Studies, ACG 2022 Annual Scientific Meeting Abstracts. Charlotte, NC: American College of Gastroenterology.
1Mayo Clinic College of Medicine and Science, Rochester, MN; 2University Hospitals Leuven, Leuven, Brabant Wallon, Belgium; 3Icahn School of Medicine at Mount Sinai, New York, NY; 4AbbVie, Inc., North Chicago, IL; 5Icahn School of Medicine, Mount Sinai, NY
Introduction: The Phase 3 maintenance study (FORTIFY) was a re-randomized responder withdrawal study that demonstrated efficacy and safety of risankizumab (RZB), an anti-p19 IL-23 antibody, versus (vs) withdrawal/placebo (PBO) in patients with moderate to severe Crohn’s disease (CD) who responded to RZB induction therapy. In this post-hoc analysis, we evaluated disease activity, as measured by Simple Endoscopic Score for CD (SES-CD), Crohn's Disease Activity Index (CDAI), and patient reported symptoms (PROs) of liquid stool frequency (SF) and abdominal pain score (APS), in patients who received up to 52 weeks (wks) of RZB or PBO in FORTIFY.
Methods: Patients enrolled in FORTIFY achieved clinical response to IV RZB induction therapy in ADVANCE and MOTIVATE studies. Clinical response was defined as ≥30% decrease in daily SF and/or ≥30% decrease in average daily APS from induction baseline [BL])both not worse than induction BL. In FORTIFY, patients were dosed Q8W with 180 mg subcutaneous (SC) RZB, 360 mg SC RZB, or PBO. For patients who entered FORTIFY, mean SES-CD and CDAI values were calculated at induction BL and at FORTIFY Wks 0, 24 (CDAI only), and 52; mean SF and APS were calculated from patient diary data at BL of induction and at Wks 8, 16, 24, 32, 40, 48, and 52 of FORTIFY.
Results: At induction BL, mean SES-CD, CDAI, SF, and AP scores were similar between treatment groups. At FORTIFY Wk 0, disease activity scores improved from induction BL for all patients, consistent with an IV RZB induction responder population for FORTIFY. Over time, patients on RZB maintenance therapy demonstrated ongoing improvement in SES-CD and CDAI values, whereas mean SES-CD and CDAI values increased in the withdrawal/PBO group. A treatment effect was evident at Wk 52. For most timepoints throughout maintenance, patients receiving RZB showed numerically lower APS and SF (RZB 360 mg only) compared to patients in the withdrawal/PBO group.
Discussion: Across the studies, RZB therapy led to marked improvements in disease activity over time and continued to show benefit compared to withdrawal/PBO at Wk 52. The pronounced benefit of RZB over withdrawal/PBO at Wk 52 for SES-CD, an objective endoscopic endpoint, contrasts with a relative lack of differentiation at Wk 52 for the subjective SF and AP endpoints. This is likely explained by residual exposure given the long half-life and pharmacodynamic effect of RZB following induction therapy, leading to prolonged symptom improvement in patients receiving PBO in maintenance.

Figure: Figure. SES-CD, CDAI, SF and AP scores over time for participants enrolled in the FORTIFY maintenance study, from induction (ADVANCE/MOTIVATE) baseline through FORTIFY Week 52
Disclosures:
Edward Loftus: AbbVie – Consultant, Grant/Research Support. Amgen – Consultant, Grant/Research Support. Arena Pharmaceuticals – Consultant. Boehringer Ingelheim – Consultant. Bristol-Myers Squibb – Consultant, Grant/Research Support. Calibr – Consultant. Celgene – Consultant, Grant/Research Support. Eli Lilly – Consultant. Genentech – Consultant, Grant/Research Support. Gilead – Consultant, Grant/Research Support. Iterative Scopes – Consultant. Janssen – Consultant, Grant/Research Support. Morphic – Consultant. Ono Pharma – Consultant. Pfizer – Consultant, Grant/Research Support. Protagonist – Consultant. Receptos – Grant/Research Support. Robarts Clinical Trials – Grant/Research Support. Scipher Medicine – Consultant. Sun Pharma – Consultant. Surrozen – Consultant. Takeda – Consultant, Grant/Research Support. Theravance – Grant/Research Support. UCB – Consultant, Grant/Research Support.
Marc Ferrante: AbbVie – Consultant, Grant/Research Support, Speakers Bureau. Amgen – Consultant, Grant/Research Support, Speakers Bureau. Biogen – Consultant, Grant/Research Support, Speakers Bureau. Boehringer Ingelheim – Consultant, Speakers Bureau. Celgene – Consultant, Speakers Bureau. Celltrion – Consultant, Speakers Bureau. Dr Falk – Consultant, Speakers Bureau. Eli Lilly – Consultant, Speakers Bureau. Ferring – Consultant, Speakers Bureau. Janssen – Grant/Research Support. Janssen-Cilag – Consultant, Speakers Bureau. Lamepro – Consultant, Speakers Bureau. Medtronic – Consultant, Speakers Bureau. Merck Sharp & Dohme – Consultant, Speakers Bureau. Mylan – Consultant, Speakers Bureau. Pfizer – Consultant, Grant/Research Support, Speakers Bureau. Regeneron – Consultant, Speakers Bureau. Samsung Bioepis – Consultant, Speakers Bureau. Sandoz – Consultant, Speakers Bureau. Takeda – Consultant, Grant/Research Support, Speakers Bureau. Thermo Fisher Scientific – Consultant, Speakers Bureau. Truvion Healthcare – Consultant, Speakers Bureau.
Jean-Frederic Colombel: AbbVie – Consultant, Grant/Research Support. Amgen – Consultant. Arena – Consultant. BMS – Consultant. Boehringer Ingelheim – Consultant. Celgene Corporation – Consultant. Celltrion – Personal Fees. Eli Lilly – Consultant. Enterome – Personal Fees. Ferring Pharmaceuticals – Consultant. Galmed Research – Consultant. Galxo Smith Kline – Consultant. Genentech – Consultant. Genfit – Stock Options. Imedex – Consultant. Immunic – Consultant. Intestinal Biotech Development – Stock Options. Intestinal Biotech Development – Stock Options. Iterative Scopes – Consultant. Janssen Pharmaceuticals – Advisory Committee/Board Member, Consultant, Grant/Research Support. Kaleido Biosciences – Consultant. MedImmune – Personal Fees. Merck – Consultant. Microba – Consultant. Novartis – Consultant. PBM Capital – Consultant. Pfizer – Consultant. PPM Services – Personal Fees. Protagonist Therapeutics – Consultant. Sanofi – Consultant. Second Genome – Personal Fees. Seres – Personal Fees. Shire – Personal Fees. Takeda – Advisory Committee/Board Member, Consultant, Grant/Research Support. Theradiag – Personal Fees. TiGenix – Consultant. Vifor – Consultant.
Kristina Kligys: AbbVie – Employee, Stock Options.
Stijn Van Haaren: AbbVie – Employee, Stock Options.
Alexandra Song: AbbVie – Employee, Stock Options.
Ezequiel Neimark: AbbVie – Employee, Stock Options.
Javier Zambrano: AbbVie – Employee, Stock Options.
Xiaomei Liao: AbbVie – Employee, Stock Options.
Marla Dubinsky: AbbVie – Consultant. Allergan – Consultant. Amgen – Consultant. Boehringer Ingelheim – Consultant. Celgene – Consultant. Eli Lilly – Consultant. Gilead – Consultant. Janssen – Consultant. Pfizer – Consultant. Prometheus Labs – Consultant. Roche – Consultant. Takeda – Consultant. Trellus Health – Share holder. UCB – Consultant.
Edward V. Loftus, MD, FACG1, Marc Ferrante, MD, PhD2, Jean-Frederic Colombel, MD3, Kristina Kligys, PhD4, Stijn Van Haaren, MsC4, Alexandra Song, MD4, Ezequiel Neimark, MD4, Javier Zambrano, MD4, Xiaomei Liao, PhD4, Marla Dubinsky, MD5. B0376 - Risankizumab Results in Improvements in Disease Activity Scores in Patients With Crohn’s Disease: Post-Hoc Analysis of the Phase 3 Induction and Maintenance Studies, ACG 2022 Annual Scientific Meeting Abstracts. Charlotte, NC: American College of Gastroenterology.
