Back

Poster Session B - Monday Morning
Category: IBD
B0384 - Pharmacokinetic Equivalence of Biosimilar Adalimumab-aqvh and Adalimumab in Healthy Subjects
Monday, October 24, 2022
10:00 AM – 12:00 PM ET
Location: Crown Ballroom

Has Audio

Suzanna Tatarewicz, MSc
Coherus Biosciences
Redwood City, CA
Presenting Author(s)
Barbara Finck, MD, Hong Tang, MS, Francesca Civoli, PhD, Suzanna Tatarewicz, MSc, Hillary O'Kelly, MPH
Coherus Biosciences, Redwood City, CA
Introduction: This study assessed the pharmacokinetic (PK) bioequivalence between adalimumab-aqvh, an FDA-approved biosimilar, and the reference product adalimumab after a single dose administered to healthy subjects. Adalimumab is indicated for many inflammatory conditions such as psoriasis as well as inflammatory bowel diseases such as ulcerative colitis and Crohn’s disease. Biosimilars are biologic drugs that are highly similar in purity, potency, efficacy, and safety to the reference biologic and are available at lower costs.
Methods: This double-blind, single-dose, parallel-group study randomized healthy subjects (N = 210) 1:1 to receive one 40-mg dose of adalimumab-aqvh or adalimumab. The primary PK endpoints were maximum serum concentrations (Cmax) and the area under the serum concentration versus time curve extrapolated from 0 to infinity (AUC0-inf) of adalimumab-aqvh versus adalimumab. Other PK endpoints included the time to reach Cmax, terminal elimination half-life, AUC calculated from 0 to the last measurable concentration, and apparent volume of serum cleared of drug per unit time. Safety and immunogenicity were also assessed.
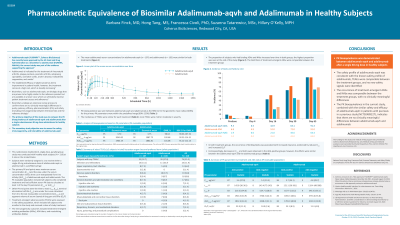
Results: The Cmax geometric mean ratio (GMR) was 98.6% (90% CI 90.7–107.3) and the AUC0-inf GMR was 102.7% (90% CI 92.2–114.3); PK bioequivalence was demonstrated, as both endpoints were contained within the predefined 90% CI GMR range (80%–125%). The other PK endpoints were similar between adalimumab-aqvh and adalimumab. Treatment-emergent adverse events (TEAEs) and study drug–related TEAEs were similar for each treatment, and most were mild or moderate in severity; no new safety signals were identified. Immunogenicity was similar after treatment with adalimumab-aqvh and adalimumab.
Discussion: The study demonstrated PK bioequivalence between adalimumab-aqvh and adalimumab following a single dose in healthy subjects. The safety profile, including immunogenicity, of adalimumab-aqvh was also similar to that of adalimumab.
Disclosures:
Barbara Finck, MD, Hong Tang, MS, Francesca Civoli, PhD, Suzanna Tatarewicz, MSc, Hillary O'Kelly, MPH. B0384 - Pharmacokinetic Equivalence of Biosimilar Adalimumab-aqvh and Adalimumab in Healthy Subjects, ACG 2022 Annual Scientific Meeting Abstracts. Charlotte, NC: American College of Gastroenterology.
Coherus Biosciences, Redwood City, CA
Introduction: This study assessed the pharmacokinetic (PK) bioequivalence between adalimumab-aqvh, an FDA-approved biosimilar, and the reference product adalimumab after a single dose administered to healthy subjects. Adalimumab is indicated for many inflammatory conditions such as psoriasis as well as inflammatory bowel diseases such as ulcerative colitis and Crohn’s disease. Biosimilars are biologic drugs that are highly similar in purity, potency, efficacy, and safety to the reference biologic and are available at lower costs.
Methods: This double-blind, single-dose, parallel-group study randomized healthy subjects (N = 210) 1:1 to receive one 40-mg dose of adalimumab-aqvh or adalimumab. The primary PK endpoints were maximum serum concentrations (Cmax) and the area under the serum concentration versus time curve extrapolated from 0 to infinity (AUC0-inf) of adalimumab-aqvh versus adalimumab. Other PK endpoints included the time to reach Cmax, terminal elimination half-life, AUC calculated from 0 to the last measurable concentration, and apparent volume of serum cleared of drug per unit time. Safety and immunogenicity were also assessed.
Results: The Cmax geometric mean ratio (GMR) was 98.6% (90% CI 90.7–107.3) and the AUC0-inf GMR was 102.7% (90% CI 92.2–114.3); PK bioequivalence was demonstrated, as both endpoints were contained within the predefined 90% CI GMR range (80%–125%). The other PK endpoints were similar between adalimumab-aqvh and adalimumab. Treatment-emergent adverse events (TEAEs) and study drug–related TEAEs were similar for each treatment, and most were mild or moderate in severity; no new safety signals were identified. Immunogenicity was similar after treatment with adalimumab-aqvh and adalimumab.
Discussion: The study demonstrated PK bioequivalence between adalimumab-aqvh and adalimumab following a single dose in healthy subjects. The safety profile, including immunogenicity, of adalimumab-aqvh was also similar to that of adalimumab.
Disclosures:
Barbara Finck: Coherus BioSciences – Consultant, Stock Options.
Hong Tang: Coherus BioSciences – Employee, Stock Options.
Francesca Civoli: Coherus BioSciences – Employee, Stock Options.
Suzanna Tatarewicz: Coherus BioSciences – Employee, Stock-publicly held company(excluding mutual/index funds).
Hillary O'Kelly: Coherus BioSciences – Employee.
Barbara Finck, MD, Hong Tang, MS, Francesca Civoli, PhD, Suzanna Tatarewicz, MSc, Hillary O'Kelly, MPH. B0384 - Pharmacokinetic Equivalence of Biosimilar Adalimumab-aqvh and Adalimumab in Healthy Subjects, ACG 2022 Annual Scientific Meeting Abstracts. Charlotte, NC: American College of Gastroenterology.
