Back

Poster Session B - Monday Morning
Category: Esophagus
B0209 - CDX2 Immunohistochemical Positivity Confirms Barrett Esophagus in the Absence of Goblet Cells and Confers Significant Risk for High Grade Dysplasia and Esophageal Adenocarcinoma: A Pilot Study
Monday, October 24, 2022
10:00 AM – 12:00 PM ET
Location: Crown Ballroom

Has Audio

Parth M. Patel, MD
Henry Ford Jackson
Jackson, MI
Presenting Author(s)
Parth M. Patel, MD1, Hassan Zreik, MD1, Merritt Bern, MD1, Douglas Grider, MD2
1Henry Ford Jackson, Jackson, MI; 2Virginia Tech Carilion School of Medicine, Roanoke, VA
Introduction: Barrett’s esophagus (BE) is a premalignant condition defined by intestinal metaplasia (IM) and can develop into esophageal adenocarcinoma (EAC). The standard tool for diagnosing BE is esophagogastroduodenoscopy (EGD). Based on American College of Gastroenterology (ACG) guidelines, patients that do not have goblet cells GCs on the initial or subsequent biopsies do not qualify for surveillance EGDs. The absence of GCs on subsequent biopsy could be due to tissue sampling error, medication effects, or true goblet cells loss over time. The caudal-related homeobox transcription factor 2 (CDX2) is a well-established marker for intestinal mucosa and is present in BE mucosa, consistent with IM. In this paper, we examine the role of CDX2 staining as a marker for BE IM when patients do not have GCs on subsequent biopsies and determine the risk for high-grade dysplasia (HGD) or EAC.
Methods: Serial biopsies of the esophagus were obtained during surveillance EGDs, with the initial biopsy set confirming BE with GCs and a second set that did not show GC. The CDX2 stains were prepared from paraffin blocks.
Results: 35 patients met all inclusion criteria and had initial biopsies that were positive for CDX2. Of those 35 patients, 34 were positive for CDX2 on subsequent slides. Of these 34 patients, one had HGD, and one had HGD and EAC. One patient was negative for GCs and CDX2 on the subsequent slides; this patient had HGD. In total, 3 of 35 patients (9%) had HGD or EAC on subsequent slides.
Discussion:
This study, while small, shows that CDX2 may be an important adjunctive test for the surveillance of BE. Our results demonstrate that BE patients with proven GCs IM on initial biopsy, but not subsequent biopsies, are overwhelmingly positive for CDX2 and still have a significant risk of HGD or EAC.
Our study has important limitations, including the small sample size due to strict inclusion criteria required by the IRB. Despite this, our study provides valuable information concerning CDX2 and the development of HGD and EAC in BE patients, especially those without GCs on subsequent biopsies.
Our study supports the use of CDX2 as an adjunctive test for the initial diagnosis of BE. More importantly, the patients with previously diagnosed BE who no longer have GCs on subsequent biopsies but are CDX2 positive continue to have a significant risk for HGD or EAC and warrant continued surveillance. We believe that CDX2 can help identify this at-risk group.
Disclosures:
Parth M. Patel, MD1, Hassan Zreik, MD1, Merritt Bern, MD1, Douglas Grider, MD2. B0209 - CDX2 Immunohistochemical Positivity Confirms Barrett Esophagus in the Absence of Goblet Cells and Confers Significant Risk for High Grade Dysplasia and Esophageal Adenocarcinoma: A Pilot Study, ACG 2022 Annual Scientific Meeting Abstracts. Charlotte, NC: American College of Gastroenterology.
1Henry Ford Jackson, Jackson, MI; 2Virginia Tech Carilion School of Medicine, Roanoke, VA
Introduction: Barrett’s esophagus (BE) is a premalignant condition defined by intestinal metaplasia (IM) and can develop into esophageal adenocarcinoma (EAC). The standard tool for diagnosing BE is esophagogastroduodenoscopy (EGD). Based on American College of Gastroenterology (ACG) guidelines, patients that do not have goblet cells GCs on the initial or subsequent biopsies do not qualify for surveillance EGDs. The absence of GCs on subsequent biopsy could be due to tissue sampling error, medication effects, or true goblet cells loss over time. The caudal-related homeobox transcription factor 2 (CDX2) is a well-established marker for intestinal mucosa and is present in BE mucosa, consistent with IM. In this paper, we examine the role of CDX2 staining as a marker for BE IM when patients do not have GCs on subsequent biopsies and determine the risk for high-grade dysplasia (HGD) or EAC.
Methods: Serial biopsies of the esophagus were obtained during surveillance EGDs, with the initial biopsy set confirming BE with GCs and a second set that did not show GC. The CDX2 stains were prepared from paraffin blocks.
Results: 35 patients met all inclusion criteria and had initial biopsies that were positive for CDX2. Of those 35 patients, 34 were positive for CDX2 on subsequent slides. Of these 34 patients, one had HGD, and one had HGD and EAC. One patient was negative for GCs and CDX2 on the subsequent slides; this patient had HGD. In total, 3 of 35 patients (9%) had HGD or EAC on subsequent slides.
Discussion:
This study, while small, shows that CDX2 may be an important adjunctive test for the surveillance of BE. Our results demonstrate that BE patients with proven GCs IM on initial biopsy, but not subsequent biopsies, are overwhelmingly positive for CDX2 and still have a significant risk of HGD or EAC.
Our study has important limitations, including the small sample size due to strict inclusion criteria required by the IRB. Despite this, our study provides valuable information concerning CDX2 and the development of HGD and EAC in BE patients, especially those without GCs on subsequent biopsies.
Our study supports the use of CDX2 as an adjunctive test for the initial diagnosis of BE. More importantly, the patients with previously diagnosed BE who no longer have GCs on subsequent biopsies but are CDX2 positive continue to have a significant risk for HGD or EAC and warrant continued surveillance. We believe that CDX2 can help identify this at-risk group.

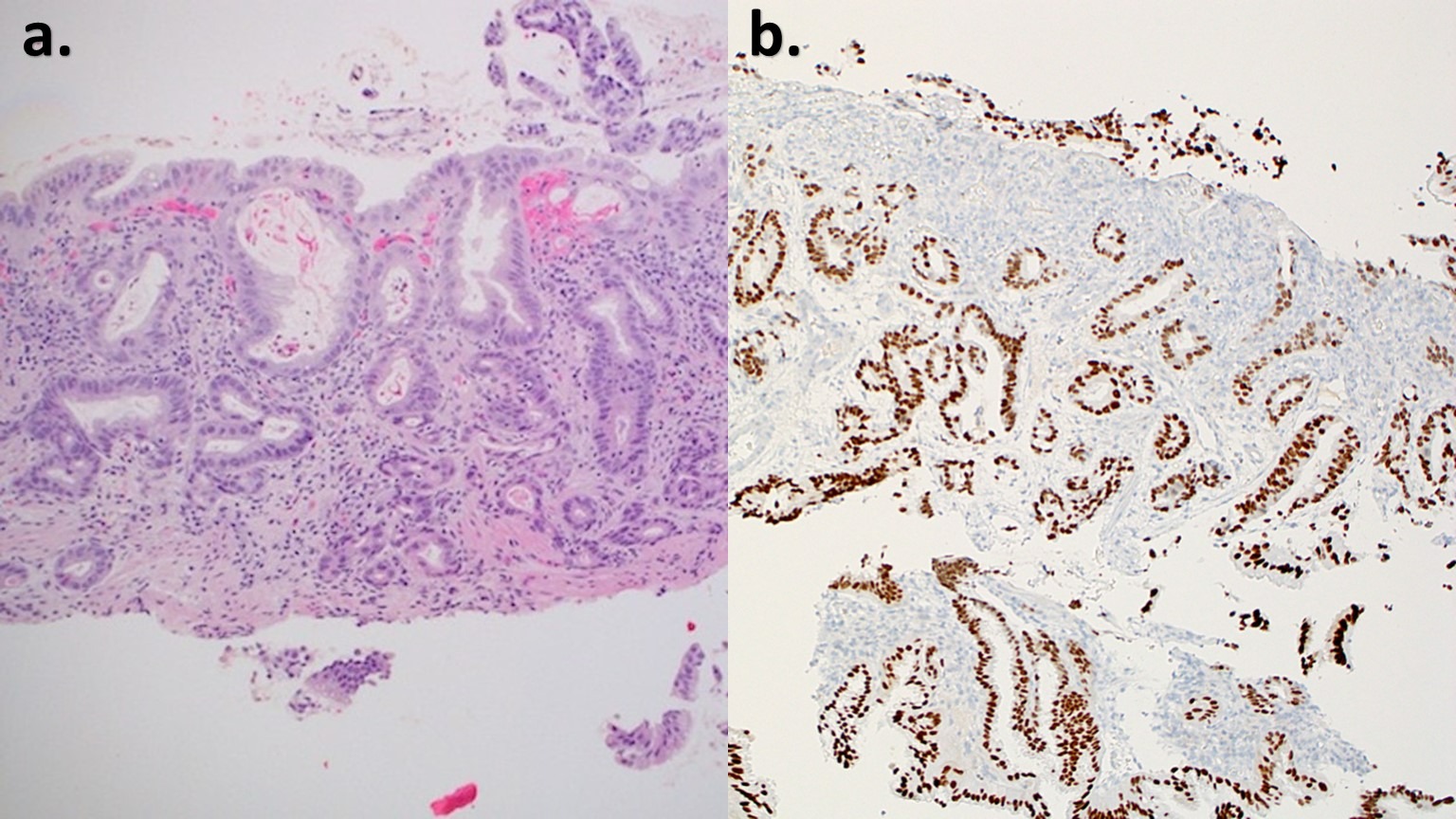
Figure: Figure 1. a. H&E stain with HGD and adenocarcinoma (102x80mm; 220 x 220 DPI) b. CDX2 Immunohistochemical stain with HGD and adenocarcinoma (101x79mm, 220 x 220 DPI)
Disclosures:
Parth Patel indicated no relevant financial relationships.
Hassan Zreik indicated no relevant financial relationships.
Merritt Bern indicated no relevant financial relationships.
Douglas Grider indicated no relevant financial relationships.
Parth M. Patel, MD1, Hassan Zreik, MD1, Merritt Bern, MD1, Douglas Grider, MD2. B0209 - CDX2 Immunohistochemical Positivity Confirms Barrett Esophagus in the Absence of Goblet Cells and Confers Significant Risk for High Grade Dysplasia and Esophageal Adenocarcinoma: A Pilot Study, ACG 2022 Annual Scientific Meeting Abstracts. Charlotte, NC: American College of Gastroenterology.

