Back

Poster Session B - Monday Morning
Category: IBD
B0377 - Mirikizumab Significantly Improves Abdominal Pain in Patients With Moderately-to-Severely Active Ulcerative Colitis: Results From the Phase 3 LUCENT-1 Induction and LUCENT-2 Maintenance Studies
Monday, October 24, 2022
10:00 AM – 12:00 PM ET
Location: Crown Ballroom

Has Audio
- EL
Edward V. Loftus, Jr., MD, FACG
Mayo Clinic College of Medicine and Science
Rochester, Minnesota
Presenting Author(s)
Edward V. Loftus, MD, FACG1, Theresa Hunter Gibble, PhD2, Alison Potts Bleakman, MA, PhD2, Xingyuan Li, PhD2, Nathan Morris, PhD2, Emily Hon, MD2, Vipul Jairath, MBChB, DPhil3
1Mayo Clinic College of Medicine and Science, Rochester, MN; 2Eli Lilly and Company, Indianapolis, IN; 3Western University, London, ON, Canada
Introduction: Abdominal pain (AP) is a frequent and burdensome symptom in patients (pts) with ulcerative colitis (UC).1 Mirikizumab (miri; IL-23p19 inhibitor) demonstrated efficacy vs placebo (PBO) in adult pts with moderately-to-severely active UC in randomized, double-blind, phase 3 LUCENT-1 (induction/NCT03518086) and LUCENT-2 (maintenance/NCT03524092) studies.2.3 Here, we report the effect of miri vs PBO on AP.
Methods: In the induction study, pts (N=1162) were randomized 3:1 to receive intravenous (IV) miri 300 mg or PBO every 4 weeks (Q4W). Pts who achieved clinical response with miri at W12 (N=544) of induction were re-randomized 2:1 to subcutaneous (SC) miri 200 mg or PBO Q4W through W40 in the maintenance study. Pts recorded “worst AP in the past 24 hours” each day using an 11-point AP Numeric Rating Scale (NRS; 0 = no pain; 10 = worst possible pain) on an electronic diary. AP improvement (AP NRS score ≥30% improvement from baseline [BL] in pts with BL AP NRS ≥3) was evaluated. The Cochran-Mantel-Haenszel test was used to compare the proportion of pts achieving AP improvement with missing data imputed as nonresponse.
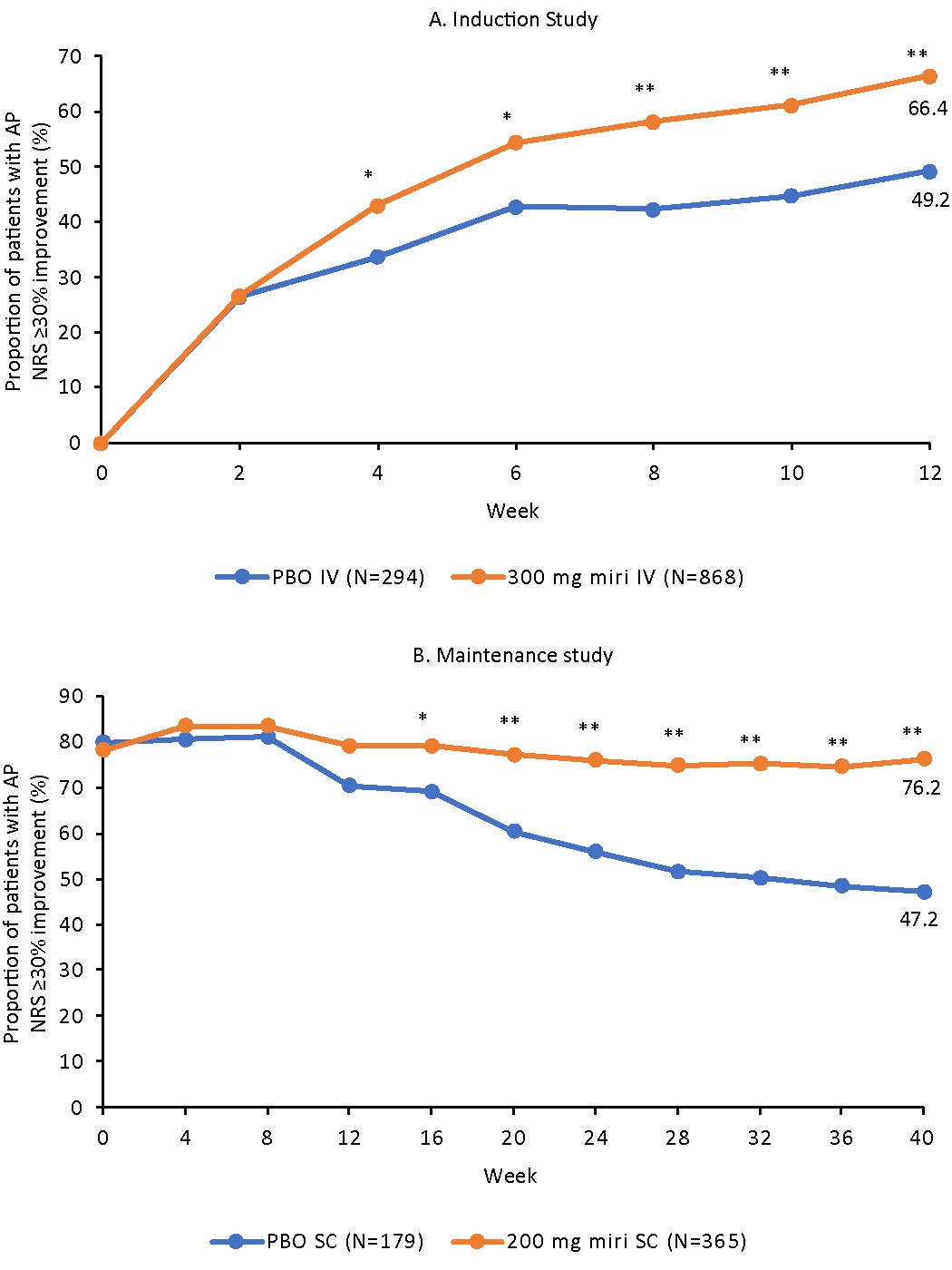
Results: As early as W4 (miri 43.0% vs PBO 33.7%; risk difference [95% CI]: 9.7 [2.8–16.6], p=0.007) of the induction study, a significant reduction from BL of at least 30% in AP NRS score was observed in the miri-treated pts vs PBO through W12 (66.4% vs 49.2%; 17.4 [10.3–24.6], p< 0.001). In the maintenance study, a greater percentage of miri-treated pts maintained AP NRS improvement compared to PBO. The separation started at W16 (79.2% vs 69.2%; 9.0 [0.5–17.5], p=0.034) and sustained through W40 (76.2% vs 47.2%; 27.4 [18.3–36.4], p< 0.001; Figure).
Discussion: Miri provided early (W4) and sustained improvement (through W40) of AP compared with PBO in pts with moderately-to-severely active UC.
1Dulai PS, et al. Development of the symptoms and impacts questionnaire for Crohn's disease and ulcerative colitis. Aliment Pharmacol Ther. 2020;51(11):1047–66.
2G D’Haens, et al. Efficacy and safety of mirikizumab as induction therapy in patients with moderately to severely active Ulcerative Colitis: Results from the Phase 3 LUCENT-1 study. J Crohn's Colitis. 2022;16:i028–29.
3M Dubinsky, et al. Efficacy and safety of mirikizumab as maintenance therapy in patients with moderately to severely active ulcerative colitis: results from the phase 3 LUCENT-2 study. Abs. 867e. Digestive Disease Week, May 21-24, 2022.
Disclosures:
Edward V. Loftus, MD, FACG1, Theresa Hunter Gibble, PhD2, Alison Potts Bleakman, MA, PhD2, Xingyuan Li, PhD2, Nathan Morris, PhD2, Emily Hon, MD2, Vipul Jairath, MBChB, DPhil3. B0377 - Mirikizumab Significantly Improves Abdominal Pain in Patients With Moderately-to-Severely Active Ulcerative Colitis: Results From the Phase 3 LUCENT-1 Induction and LUCENT-2 Maintenance Studies, ACG 2022 Annual Scientific Meeting Abstracts. Charlotte, NC: American College of Gastroenterology.
1Mayo Clinic College of Medicine and Science, Rochester, MN; 2Eli Lilly and Company, Indianapolis, IN; 3Western University, London, ON, Canada
Introduction: Abdominal pain (AP) is a frequent and burdensome symptom in patients (pts) with ulcerative colitis (UC).1 Mirikizumab (miri; IL-23p19 inhibitor) demonstrated efficacy vs placebo (PBO) in adult pts with moderately-to-severely active UC in randomized, double-blind, phase 3 LUCENT-1 (induction/NCT03518086) and LUCENT-2 (maintenance/NCT03524092) studies.2.3 Here, we report the effect of miri vs PBO on AP.
Methods: In the induction study, pts (N=1162) were randomized 3:1 to receive intravenous (IV) miri 300 mg or PBO every 4 weeks (Q4W). Pts who achieved clinical response with miri at W12 (N=544) of induction were re-randomized 2:1 to subcutaneous (SC) miri 200 mg or PBO Q4W through W40 in the maintenance study. Pts recorded “worst AP in the past 24 hours” each day using an 11-point AP Numeric Rating Scale (NRS; 0 = no pain; 10 = worst possible pain) on an electronic diary. AP improvement (AP NRS score ≥30% improvement from baseline [BL] in pts with BL AP NRS ≥3) was evaluated. The Cochran-Mantel-Haenszel test was used to compare the proportion of pts achieving AP improvement with missing data imputed as nonresponse.
Results: As early as W4 (miri 43.0% vs PBO 33.7%; risk difference [95% CI]: 9.7 [2.8–16.6], p=0.007) of the induction study, a significant reduction from BL of at least 30% in AP NRS score was observed in the miri-treated pts vs PBO through W12 (66.4% vs 49.2%; 17.4 [10.3–24.6], p< 0.001). In the maintenance study, a greater percentage of miri-treated pts maintained AP NRS improvement compared to PBO. The separation started at W16 (79.2% vs 69.2%; 9.0 [0.5–17.5], p=0.034) and sustained through W40 (76.2% vs 47.2%; 27.4 [18.3–36.4], p< 0.001; Figure).
Discussion: Miri provided early (W4) and sustained improvement (through W40) of AP compared with PBO in pts with moderately-to-severely active UC.
1Dulai PS, et al. Development of the symptoms and impacts questionnaire for Crohn's disease and ulcerative colitis. Aliment Pharmacol Ther. 2020;51(11):1047–66.
2G D’Haens, et al. Efficacy and safety of mirikizumab as induction therapy in patients with moderately to severely active Ulcerative Colitis: Results from the Phase 3 LUCENT-1 study. J Crohn's Colitis. 2022;16:i028–29.
3M Dubinsky, et al. Efficacy and safety of mirikizumab as maintenance therapy in patients with moderately to severely active ulcerative colitis: results from the phase 3 LUCENT-2 study. Abs. 867e. Digestive Disease Week, May 21-24, 2022.

Figure: Figure. The proportion of patients with AP NRS ≥30% improvement at A. induction and B. maintenance in patients with Abdominal Pain NRS score ≥3 at induction baseline. *p<0.05; **p<0.001 vs. placebo
Weekly measures were calculated by averaging data from daily diary entries of AP NRS for a 7-day period. Baseline value for both induction and maintenance was calculated from daily diary entries the week prior to W0 of induction.
Abbreviations: AP NRS, Abdominal Pain Numeric Rating Scale; IV, intravenous; miri, mirikizumab; PBO, placebo; SC, subcutaneous
Weekly measures were calculated by averaging data from daily diary entries of AP NRS for a 7-day period. Baseline value for both induction and maintenance was calculated from daily diary entries the week prior to W0 of induction.
Abbreviations: AP NRS, Abdominal Pain Numeric Rating Scale; IV, intravenous; miri, mirikizumab; PBO, placebo; SC, subcutaneous
Disclosures:
Edward Loftus: AbbVie – Consultant, Grant/Research Support. Amgen – Consultant, Grant/Research Support. Arena Pharmaceuticals – Consultant. Boehringer Ingelheim – Consultant. Bristol-Myers Squibb – Consultant, Grant/Research Support. Calibr – Consultant. Celgene – Consultant, Grant/Research Support. Eli Lilly – Consultant. Genentech – Consultant, Grant/Research Support. Gilead – Consultant, Grant/Research Support. Iterative Scopes – Consultant. Janssen – Consultant, Grant/Research Support. Morphic – Consultant. Ono Pharma – Consultant. Pfizer – Consultant, Grant/Research Support. Protagonist – Consultant. Receptos – Grant/Research Support. Robarts Clinical Trials – Grant/Research Support. Scipher Medicine – Consultant. Sun Pharma – Consultant. Surrozen – Consultant. Takeda – Consultant, Grant/Research Support. Theravance – Grant/Research Support. UCB – Consultant, Grant/Research Support.
Theresa Hunter Gibble: Eli Lilly and Company – Employee.
Alison Potts Bleakman: Eli Lilly and Company – Employee.
Xingyuan Li: Eli Lilly and Company – Employee.
Nathan Morris: Eli Lilly and Company – Employee.
Emily Hon: Eli Lilly and Company – Employee.
Vipul Jairath: AbbVie – consulting/advisory board fees, speaker’s fees. Alimentiv Inc – Consulting/advisory board fees. Arena pharmaceuticals – consulting/advisory board fees. Asahi Kasei Pharma – Consulting/advisory board fees. Asieris – consulting/advisory board fees. AstraZeneca – consulting/advisory board fees. Bristol Myers Squibb – consulting/advisory board fees, speaker’s fees. Celltrion – consulting/advisory board fees. Eli Lilly and Company – consulting/advisory board fees. Ferring – consulting/advisory board fees, speaker's fees. Flagship Pioneering – consulting/advisory board fees. Fresenius Kabi – consulting/advisory board fees, speaker’s fees. Galapagos – consulting/advisory board fees, speaker’s fees. Genentech – consulting/advisory board fees. Gilead – consulting/advisory board fees. GlaxoSmithKline – consulting/advisory board fees. Janssen – consulting/advisory board fees, speaker’s fees. Merck – consulting/advisory board fees. Metacrine – consulting/advisory board fees. Mylan – consulting/advisory board fees. Pandion – consulting/advisory board fees. Pendopharm – consulting/advisory board fees. Pfizer – consulting/advisory board fees, speaker’s fees. Prometheus – consulting/advisory board fees. Protagonist Therapeutics – consulting/advisory board fees. Reistone Biopharma – consulting/advisory board fees. Roche – consulting/advisory board fees. Sandoz – consulting/advisory board fees. Second Genome – consulting/advisory board fees. Shire – speaker’s fees. Sorriso pharmaceuticals – consulting/advisory board fees. Takeda – consulting/advisory board fees, speaker’s fees. Teva – consulting/advisory board fees. Topivert – consulting/advisory board fees. Ventyx Biosciences – consulting/advisory board fees. Vividion Therapeutics – consulting/advisory board fees.
Edward V. Loftus, MD, FACG1, Theresa Hunter Gibble, PhD2, Alison Potts Bleakman, MA, PhD2, Xingyuan Li, PhD2, Nathan Morris, PhD2, Emily Hon, MD2, Vipul Jairath, MBChB, DPhil3. B0377 - Mirikizumab Significantly Improves Abdominal Pain in Patients With Moderately-to-Severely Active Ulcerative Colitis: Results From the Phase 3 LUCENT-1 Induction and LUCENT-2 Maintenance Studies, ACG 2022 Annual Scientific Meeting Abstracts. Charlotte, NC: American College of Gastroenterology.
