Back

Poster Session B - Monday Morning
Category: IBD
B0402 - A Scintigraphic Study to Evaluate the Safety, Tolerability, and Functions of a Drug Delivery System (DDS) Device in Healthy Subjects in Fasted State
Monday, October 24, 2022
10:00 AM – 12:00 PM ET
Location: Crown Ballroom

Has Audio
- SL
Shaoying N. Lee, PhD
Biora Therapeutics
San Diego, CA
Presenting Author(s)
Shaoying N. Lee, PhD1, Erik Sandefer, PhD2, Cheryl Stork, PhD1, Walter Doll, PhD, RPH2, Richard C. Page, PhD2, Nelson Quintana, BA1, Chris Wahl, MD, PhD1, Sharat Singh, PhD1
1Biora Therapeutics, San Diego, CA; 2Scintipharma, Inc., Lexington, KY
Introduction: The clinical remission in moderate to severe ulcerative colitis (UC) and Crohn’s disease has plateaued at ~15-20% even with the approval of multiple biologics/drugs. The ATLAS study demonstrated that the lack of adequate amount of drug at the diseased site is responsible for limited clinical benefit. The Drug Delivery System (DDS) is an ingestible electronic targeted delivery device containing a localization system to identify colon entry (S4 calls) based on the gastrointestinal (GI) anatomy and deliver a bolus dose of a therapeutic compound to the colon mucosa to improve efficacy and reduce systemic toxicity. This was an open-label and single-center study to evaluate the safety and tolerability, and functions of a DDS device using gamma scintigraphy in normal healthy volunteers (NHV) in the fasted state.
Methods: Each subject was fasted overnight for a minimum of 8 hrs. and dosed with a single DDS device before resuming a normal diet at 4 hrs. post-dosing. Each capsule was filled with radioactive marker 111In-DTPA to identify DDS localization and to visualize payload release; water radiolabeled with 99mTc-DTPA was co-administered with the DDS to delineate GI landmarks by gamma scintigraphy. The GI transit of DDS and delivery location were confirmed by serial scintigraphy imaging and compared with the localization data in the recovered capsule.
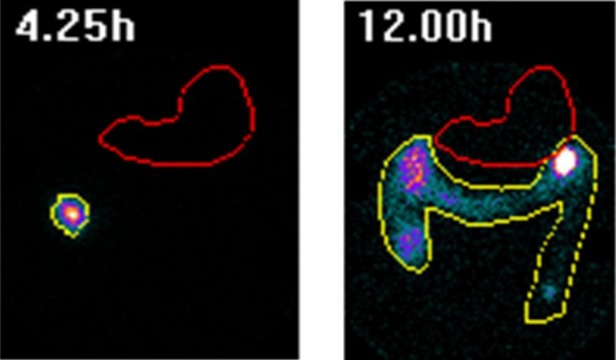
Results: Twelve male subjects were enrolled and treated in the study. There were no reported device-related adverse events in any subject who completed the study. GI transit metrics of DDS were consistent with GI residence times observed amongst NHV. There was no early release of the radio payload prior to S4 calls being made by all 12 devices. Ten of twelve devices (83%) showed correct localization calls in the colon, confirmed by scintigraphy image analysis. In addition, among devices that showed release of the 111In-DTPA payload, the dispersion of the radioactive marker completely covered the colon over time and spread to match the 99mTc-DTPA water coverage area from the site of DDS release throughout the remainder of the colon (Fig. 1).
Discussion: This study demonstrated that DDS was well-tolerated and had a favorable safety profile, and the device functioned as intended in identifying and releasing drug payload in the colon. DDS provides more precise dosing with a liquid formulation at a pre-determined location in the GIT and delivers therapeutics to the site of disease to improve efficacy.
Disclosures:
Shaoying N. Lee, PhD1, Erik Sandefer, PhD2, Cheryl Stork, PhD1, Walter Doll, PhD, RPH2, Richard C. Page, PhD2, Nelson Quintana, BA1, Chris Wahl, MD, PhD1, Sharat Singh, PhD1. B0402 - A Scintigraphic Study to Evaluate the Safety, Tolerability, and Functions of a Drug Delivery System (DDS) Device in Healthy Subjects in Fasted State, ACG 2022 Annual Scientific Meeting Abstracts. Charlotte, NC: American College of Gastroenterology.
1Biora Therapeutics, San Diego, CA; 2Scintipharma, Inc., Lexington, KY
Introduction: The clinical remission in moderate to severe ulcerative colitis (UC) and Crohn’s disease has plateaued at ~15-20% even with the approval of multiple biologics/drugs. The ATLAS study demonstrated that the lack of adequate amount of drug at the diseased site is responsible for limited clinical benefit. The Drug Delivery System (DDS) is an ingestible electronic targeted delivery device containing a localization system to identify colon entry (S4 calls) based on the gastrointestinal (GI) anatomy and deliver a bolus dose of a therapeutic compound to the colon mucosa to improve efficacy and reduce systemic toxicity. This was an open-label and single-center study to evaluate the safety and tolerability, and functions of a DDS device using gamma scintigraphy in normal healthy volunteers (NHV) in the fasted state.
Methods: Each subject was fasted overnight for a minimum of 8 hrs. and dosed with a single DDS device before resuming a normal diet at 4 hrs. post-dosing. Each capsule was filled with radioactive marker 111In-DTPA to identify DDS localization and to visualize payload release; water radiolabeled with 99mTc-DTPA was co-administered with the DDS to delineate GI landmarks by gamma scintigraphy. The GI transit of DDS and delivery location were confirmed by serial scintigraphy imaging and compared with the localization data in the recovered capsule.
Results: Twelve male subjects were enrolled and treated in the study. There were no reported device-related adverse events in any subject who completed the study. GI transit metrics of DDS were consistent with GI residence times observed amongst NHV. There was no early release of the radio payload prior to S4 calls being made by all 12 devices. Ten of twelve devices (83%) showed correct localization calls in the colon, confirmed by scintigraphy image analysis. In addition, among devices that showed release of the 111In-DTPA payload, the dispersion of the radioactive marker completely covered the colon over time and spread to match the 99mTc-DTPA water coverage area from the site of DDS release throughout the remainder of the colon (Fig. 1).
Discussion: This study demonstrated that DDS was well-tolerated and had a favorable safety profile, and the device functioned as intended in identifying and releasing drug payload in the colon. DDS provides more precise dosing with a liquid formulation at a pre-determined location in the GIT and delivers therapeutics to the site of disease to improve efficacy.

Figure: Figure 1. Cumulative distribution of radiotracer 111In-DTPA release from Drug Delivery System (DDS) post-dose A. the first release of 111In-DTPA was observed post-dose in the cecum/ascending colon. B. Distribution of released 111In-DTPA over time in the colon.
Disclosures:
Shaoying Lee: Biora Therapeutics – Employee. crohn's and colitis foundation – Grant/Research Support.
Erik Sandefer: Biora Therapeutics – Independent Contractor. Scintipharma, Inc. – Employee, Owner/Ownership Interest, Stock-privately held company.
Cheryl Stork: Biora Therapeutics – Employee.
Walter Doll: Biora Therapeutics, Inc. – Independent Contractor.
Richard Page: Biora Therapeutics, Inc. – Independent Contractor. Scintipharma, Inc. – Employee.
Nelson Quintana: Biora Therapeutics – Employee.
Chris Wahl: Biora Therapeutics – Employee.
Sharat Singh: Bioratherapeutics – Employee.
Shaoying N. Lee, PhD1, Erik Sandefer, PhD2, Cheryl Stork, PhD1, Walter Doll, PhD, RPH2, Richard C. Page, PhD2, Nelson Quintana, BA1, Chris Wahl, MD, PhD1, Sharat Singh, PhD1. B0402 - A Scintigraphic Study to Evaluate the Safety, Tolerability, and Functions of a Drug Delivery System (DDS) Device in Healthy Subjects in Fasted State, ACG 2022 Annual Scientific Meeting Abstracts. Charlotte, NC: American College of Gastroenterology.
