Back

Poster Session B - Monday Morning
Category: IBD
B0406 - Mirikizumab Demonstrates Sustained Improvement in Fatigue in Patients with Moderately-to-Severely Active Ulcerative Colitis: Results From the Phase 3 LUCENT-1 Induction and LUCENT-2 Maintenance Studies
Monday, October 24, 2022
10:00 AM – 12:00 PM ET
Location: Crown Ballroom

Has Audio

Miguel Regueiro, MD
Cleveland Clinic Main Campus
Cleveland, Ohio
Presenting Author(s)
Miguel Regueiro, MD1, Theresa Hunter Gibble, PhD2, Alison Potts Bleakman, MA, PhD2, Xingyuan Li, PhD2, Nathan Morris, PhD2, William J. Eastman, MD2
1Cleveland Clinic Main Campus, Cleveland, OH; 2Eli Lilly and Company, Indianapolis, IN
Introduction: Fatigue is a significant concern for patients (pts) with ulcerative colitis (UC).1 Mirikizumab (miri), an anti-IL-23p19 monoclonal antibody, demonstrated efficacy vs placebo (PBO) in adult pts with moderately-to-severely active UC in phase 3, randomized, double-blind, PBO-controlled 12-week (W) induction (LUCENT-1/NCT03518086) and 40-W maintenance (LUCENT-2/NCT03524092) studies.2,3 Here, we report the effect of miri vs PBO on fatigue.
Methods: In the induction study, pts (N=1162) were randomized (3:1) to intravenous (IV) miri 300 mg or PBO every 4 weeks (Q4W). Pts who achieved clinical response with miri at W12 (N=544) in the induction were re-randomized (2:1) to subcutaneous (SC) miri 200 mg or PBO Q4W through W40 in the maintenance study. Fatigue was measured using the Fatigue Numeric Rating Scale (NRS), a single-question patient-reported instrument that measures the “worst fatigue in the past 24 hours” with an 11-point NRS (0 = no fatigue, 10 = fatigue as bad as you can imagine), on an electronic daily diary in LUCENT-1 and W40 of LUCENT-2 studies. The treatment difference in least squares mean (LSM) change from the baseline (W0 of therapy) in Fatigue NRS scores was determined using the analysis of covariance model.
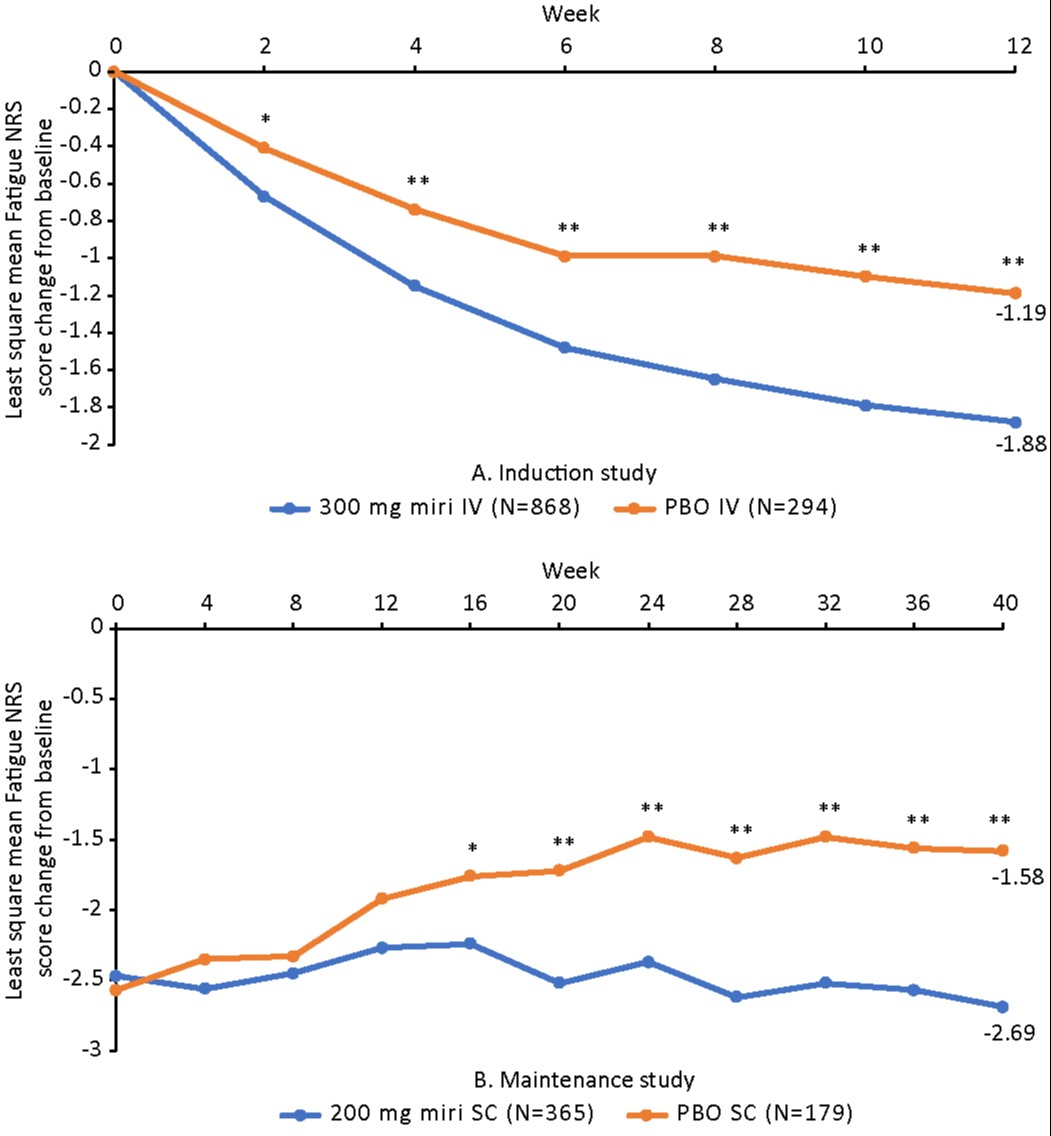
Results: In the induction study, a significant reduction in Fatigue NRS score vs PBO was observed as early as W2 (LSM difference [95% CI]: −0.25 [−0.45, −0.05], p=0.013). The LSM difference from baseline at W12 was −0.69 (−0.98, −0.40; p< 0.001). In the maintenance study, a significant reduction in Fatigue NRS score compared to PBO was observed from W16 and sustained through W40 (−1.10 [−1.53, −0.67]; p< 0.001; Figure).
Discussion: Miri-treated pts with moderately-to-severely active UC showed early (W2) and sustained (W40) improvements in fatigue compared to PBO.
1Huppertz-Hauss G, et al. Fatigue in a population-based cohort of patients with inflammatory bowel disease 20 years after diagnosis: the IBSEN study. Scand J Gastroenterol. 2017;52:351–358.
2G D’Haens, et al. Efficacy and safety of mirikizumab as induction therapy in patients with moderately to severely active Ulcerative Colitis: Results from the Phase 3 LUCENT-1 study. J Crohn's Colitis. 2022;16:i028–29.
3M Dubinsky, et al. Efficacy and safety of mirikizumab as maintenance therapy in patients with moderately to severely active ulcerative colitis: results from the phase 3 LUCENT-2 study. Abs. 867e. Digestive Disease Week, May 21-24, 2022.
Disclosures:
Miguel Regueiro, MD1, Theresa Hunter Gibble, PhD2, Alison Potts Bleakman, MA, PhD2, Xingyuan Li, PhD2, Nathan Morris, PhD2, William J. Eastman, MD2. B0406 - Mirikizumab Demonstrates Sustained Improvement in Fatigue in Patients with Moderately-to-Severely Active Ulcerative Colitis: Results From the Phase 3 LUCENT-1 Induction and LUCENT-2 Maintenance Studies, ACG 2022 Annual Scientific Meeting Abstracts. Charlotte, NC: American College of Gastroenterology.
1Cleveland Clinic Main Campus, Cleveland, OH; 2Eli Lilly and Company, Indianapolis, IN
Introduction: Fatigue is a significant concern for patients (pts) with ulcerative colitis (UC).1 Mirikizumab (miri), an anti-IL-23p19 monoclonal antibody, demonstrated efficacy vs placebo (PBO) in adult pts with moderately-to-severely active UC in phase 3, randomized, double-blind, PBO-controlled 12-week (W) induction (LUCENT-1/NCT03518086) and 40-W maintenance (LUCENT-2/NCT03524092) studies.2,3 Here, we report the effect of miri vs PBO on fatigue.
Methods: In the induction study, pts (N=1162) were randomized (3:1) to intravenous (IV) miri 300 mg or PBO every 4 weeks (Q4W). Pts who achieved clinical response with miri at W12 (N=544) in the induction were re-randomized (2:1) to subcutaneous (SC) miri 200 mg or PBO Q4W through W40 in the maintenance study. Fatigue was measured using the Fatigue Numeric Rating Scale (NRS), a single-question patient-reported instrument that measures the “worst fatigue in the past 24 hours” with an 11-point NRS (0 = no fatigue, 10 = fatigue as bad as you can imagine), on an electronic daily diary in LUCENT-1 and W40 of LUCENT-2 studies. The treatment difference in least squares mean (LSM) change from the baseline (W0 of therapy) in Fatigue NRS scores was determined using the analysis of covariance model.
Results: In the induction study, a significant reduction in Fatigue NRS score vs PBO was observed as early as W2 (LSM difference [95% CI]: −0.25 [−0.45, −0.05], p=0.013). The LSM difference from baseline at W12 was −0.69 (−0.98, −0.40; p< 0.001). In the maintenance study, a significant reduction in Fatigue NRS score compared to PBO was observed from W16 and sustained through W40 (−1.10 [−1.53, −0.67]; p< 0.001; Figure).
Discussion: Miri-treated pts with moderately-to-severely active UC showed early (W2) and sustained (W40) improvements in fatigue compared to PBO.
1Huppertz-Hauss G, et al. Fatigue in a population-based cohort of patients with inflammatory bowel disease 20 years after diagnosis: the IBSEN study. Scand J Gastroenterol. 2017;52:351–358.
2G D’Haens, et al. Efficacy and safety of mirikizumab as induction therapy in patients with moderately to severely active Ulcerative Colitis: Results from the Phase 3 LUCENT-1 study. J Crohn's Colitis. 2022;16:i028–29.
3M Dubinsky, et al. Efficacy and safety of mirikizumab as maintenance therapy in patients with moderately to severely active ulcerative colitis: results from the phase 3 LUCENT-2 study. Abs. 867e. Digestive Disease Week, May 21-24, 2022.

Figure: Figure. Change from Baseline in Fatigue NRS during A. Induction and B. Maintenance. *p<0.05; **p<0.001 vs placebo
Fatigue NRS score was collected as a single measurement at each visit from W4 to W36 in LUCENT-2.
Weekly measures were calculated by averaging data from all available daily diary entries of Fatigue NRS scores for a 7-day period.
The baseline value for both induction and maintenance was calculated from daily diary entries the week prior to W0 of induction.
Abbreviations: NRS, Numeric Rating Scale; miri, mirikizumab; PBO, placebo; IV, intravenous; SC, subcutaneous
Fatigue NRS score was collected as a single measurement at each visit from W4 to W36 in LUCENT-2.
Weekly measures were calculated by averaging data from all available daily diary entries of Fatigue NRS scores for a 7-day period.
The baseline value for both induction and maintenance was calculated from daily diary entries the week prior to W0 of induction.
Abbreviations: NRS, Numeric Rating Scale; miri, mirikizumab; PBO, placebo; IV, intravenous; SC, subcutaneous
Disclosures:
Miguel Regueiro: Abbvie – Advisory Committee/Board Member, Consultant. Abbvie – Grant/Research Support. ALFASIGMA, S.p.A. – Advisory Committee/Board Member. Allergan – Advisory Committee/Board Member. Amgen – Advisory Committee/Board Member. BMS – Grant/Research Support. Bristol Meyer Squibb – Advisory Committee/Board Member, Consultant. CC360 – Royalties. Celgene – Advisory Committee/Board Member. CME Outfitters – CME Companies. Cornerstones – CME Companies. Crohn’s and Colitis Foundation as Editor-in-Chief – Royalties. Genentech – Advisory Committee/Board Member. Genentech – Grant/Research Support. Gilead – Advisory Committee/Board Member. Gilead – Grant/Research Support. HMPGlobal – CME Companies. Imedex, GI Health Foundation (GiHF) – CME Companies. Janssen – Advisory Committee/Board Member, Consultant. Janssen – Grant/Research Support. Lilly – Grant/Research Support. MDEducation – CME Companies. Medscape – CME Companies. Miraca Labs – Advisory Committee/Board Member. MJH life sciences – CME Companies. Pfizer – Advisory Committee/Board Member, Consultant. Prometheus – Advisory Committee/Board Member. Remedy – CME Companies. Salix – Advisory Committee/Board Member. Seres – Advisory Committee/Board Member. Takeda – Advisory Committee/Board Member, Consultant. TARGET Pharma Solutions – Advisory Committee/Board Member. UCB – Grant/Research Support. WebMD – CME Companies. Wolters Kluwer Health as Author/Editor of UpToDate – Royalties.
Theresa Hunter Gibble: Eli Lilly and Company – Employee.
Alison Potts Bleakman: Eli Lilly and Company – Employee.
Xingyuan Li: Eli Lilly and Company – Employee.
Nathan Morris: Eli Lilly and Company – Employee.
William Eastman: Eli Lilly and Company – Employee.
Miguel Regueiro, MD1, Theresa Hunter Gibble, PhD2, Alison Potts Bleakman, MA, PhD2, Xingyuan Li, PhD2, Nathan Morris, PhD2, William J. Eastman, MD2. B0406 - Mirikizumab Demonstrates Sustained Improvement in Fatigue in Patients with Moderately-to-Severely Active Ulcerative Colitis: Results From the Phase 3 LUCENT-1 Induction and LUCENT-2 Maintenance Studies, ACG 2022 Annual Scientific Meeting Abstracts. Charlotte, NC: American College of Gastroenterology.
