Back

Poster Session B - Monday Morning
Category: IBD
B0415 - The Impact of Immunosuppression for Immune Checkpoint Inhibitor Colitis on Cancer Outcome
Monday, October 24, 2022
10:00 AM – 12:00 PM ET
Location: Crown Ballroom

Has Audio
- MS
Malek Shatila, MD
MD Anderson Cancer Center
Houston, TX
Presenting Author(s)
Malek Shatila, MD1, Yinghong Wang, MD, PhD1, Weije Ma, MD2, Yantong Cui, 3, Anusha S. Thomas, MD1
1MD Anderson Cancer Center, Houston, TX; 2Zhongnan Hospital of Wuhan University, Wuhan, Hubei, China; 3Cornell University, Ithaca, NY
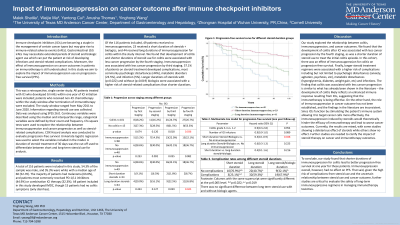
Introduction: Immune checkpoint inhibitors are becoming a staple in the management of certain types of cancer. However, they may give rise to immune-related adverse events (irAEs). In particular, gastrointestinal irAEs may sometimes necessitate extended periods of steroid use and initiation of biologic agents. In this study, we aim to explore the impact of immunosuppression use and duration on cancer progression and progression-free survival (PFS).
Methods: This is a single center retrospective review in patients taking ICIs who developed gastrointestinal irAEs within one year of ICI initiation. The study window for data collection ranged from May 2011 to June 2020. Data was analyzed using IBM SPSS Statistics 26; univariate logistic regression was used to explore the relationship between immunosuppression and cancer progression and COX Hazard analysis was conducted to evaluate progression-free survival. 30 days was used as the cut-off to differentiate between short and long-term steroid use for analysis.
Results: 113 patients were included in this study, 49 of whom did not receive any immunosuppression. All patients developed IMDC, but 16 patients had no colitis symptoms. 23 patients received a short duration of steroids while 41 patients received immunosuppression for longer than 30 days. The development of colitis was associated with less cancer progression by the fourth staging (p< 0.05) within the study window while immunosuppression for colitis correlated with less progression by third staging (p< 0.05). The multivariate COX analysis found that durations of steroid use (with or without concurrent biologic use) less than 30 days were associated with better progression-free survival (PFS;p=0.048). The number of ICI infusions also seemed to correlate with better PFS (p=0.089).
Discussion: The impact of immunosuppressive treatment on cancer outcomes has not been well studied. This study found that durations of steroid treatment less than 30 days for ICI colitis showed significantly less cancer progression within one year of ICI treatment, supporting emerging evidence that prolonged steroid use may interfere with immunotherapy efficacy. We also found that the development of colitis and the use of immunosuppression was associated with less cancer progression with a year. This study supports the benefit of GI irAE on cancer outcome and raises concern for the safety of long-term immunosuppression for managing ICI GI toxicities highlighting the need for more extensive research into this particular area.
Disclosures:
Malek Shatila, MD1, Yinghong Wang, MD, PhD1, Weije Ma, MD2, Yantong Cui, 3, Anusha S. Thomas, MD1. B0415 - The Impact of Immunosuppression for Immune Checkpoint Inhibitor Colitis on Cancer Outcome, ACG 2022 Annual Scientific Meeting Abstracts. Charlotte, NC: American College of Gastroenterology.
1MD Anderson Cancer Center, Houston, TX; 2Zhongnan Hospital of Wuhan University, Wuhan, Hubei, China; 3Cornell University, Ithaca, NY
Introduction: Immune checkpoint inhibitors are becoming a staple in the management of certain types of cancer. However, they may give rise to immune-related adverse events (irAEs). In particular, gastrointestinal irAEs may sometimes necessitate extended periods of steroid use and initiation of biologic agents. In this study, we aim to explore the impact of immunosuppression use and duration on cancer progression and progression-free survival (PFS).
Methods: This is a single center retrospective review in patients taking ICIs who developed gastrointestinal irAEs within one year of ICI initiation. The study window for data collection ranged from May 2011 to June 2020. Data was analyzed using IBM SPSS Statistics 26; univariate logistic regression was used to explore the relationship between immunosuppression and cancer progression and COX Hazard analysis was conducted to evaluate progression-free survival. 30 days was used as the cut-off to differentiate between short and long-term steroid use for analysis.
Results: 113 patients were included in this study, 49 of whom did not receive any immunosuppression. All patients developed IMDC, but 16 patients had no colitis symptoms. 23 patients received a short duration of steroids while 41 patients received immunosuppression for longer than 30 days. The development of colitis was associated with less cancer progression by the fourth staging (p< 0.05) within the study window while immunosuppression for colitis correlated with less progression by third staging (p< 0.05). The multivariate COX analysis found that durations of steroid use (with or without concurrent biologic use) less than 30 days were associated with better progression-free survival (PFS;p=0.048). The number of ICI infusions also seemed to correlate with better PFS (p=0.089).
Discussion: The impact of immunosuppressive treatment on cancer outcomes has not been well studied. This study found that durations of steroid treatment less than 30 days for ICI colitis showed significantly less cancer progression within one year of ICI treatment, supporting emerging evidence that prolonged steroid use may interfere with immunotherapy efficacy. We also found that the development of colitis and the use of immunosuppression was associated with less cancer progression with a year. This study supports the benefit of GI irAE on cancer outcome and raises concern for the safety of long-term immunosuppression for managing ICI GI toxicities highlighting the need for more extensive research into this particular area.
Disclosures:
Malek Shatila indicated no relevant financial relationships.
Yinghong Wang: AzurRx – Consultant. MabQuest – Advisory Committee/Board Member. Sorriso – Consultant. Tillotts – Consultant.
Weije Ma indicated no relevant financial relationships.
Yantong Cui indicated no relevant financial relationships.
Anusha Thomas indicated no relevant financial relationships.
Malek Shatila, MD1, Yinghong Wang, MD, PhD1, Weije Ma, MD2, Yantong Cui, 3, Anusha S. Thomas, MD1. B0415 - The Impact of Immunosuppression for Immune Checkpoint Inhibitor Colitis on Cancer Outcome, ACG 2022 Annual Scientific Meeting Abstracts. Charlotte, NC: American College of Gastroenterology.
