Back

Poster Session A - Sunday Afternoon
Category: Stomach
A0685 - Pooled Efficacy Analysis of Tradipitant in Idiopathic and Diabetic Gastroparesis Study VP-VLY-686-3301 and VP-VLY-686-2301
Sunday, October 23, 2022
5:00 PM – 7:00 PM ET
Location: Crown Ballroom

Has Audio

Jesse L. Carlin, PhD
Vanda Pharmaceuticals, Inc.
Washington, DC
Presenting Author(s)
Award: Presidential Poster Award
Jesse L. Carlin, PhD, Christos Polymeropoulos, MD, Michaela Fisher, BS, Darby Madonick, BA, Caleigh Kupersmith, BA, Paula Moszczynski, MS, Changfu Xiao, PhD, Gunther Birznieks, MS, Mihael H. Polymeropoulos, MD
Vanda Pharmaceuticals, Inc., Washington, DC
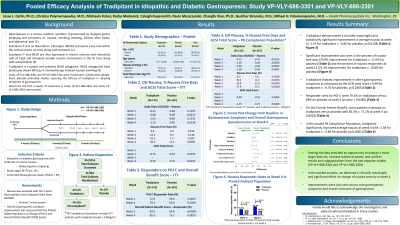
Introduction: Tradipitant is a novel NK-1 receptor antagonist studied in diabetic and idiopathic gastroparesis for short-term relief of nausea. This report presents the pooled analysis from 2 multicenter, randomized, double-blind, placebo-controlled studies (VP-VLY-686-3301 and VP-VLY-686-2301) assessing the efficacy of tradipitant in relieving symptoms of gastroparesis.
Methods: N=342 idiopathic and diabetic gastroparesis patients with delayed gastric emptying, moderate to severe nausea were included in the pooled ITT (Intent-to-Treat) population. Subjects were randomized to 85mg tradipitant twice a day (n=175) or placebo (n=167) and endpoints were assessed at Week 4. Nausea was assessed with the 5-point Gastroparesis Core Symptom Daily Diary (GCSDD). Overall gastroparesis symptom improvement was evaluated with the Patient Global Impression of Change (PGI-C) and Overall Patient Benefit (OPB) scales. Sensitivity analyses were performed to control for confounders.
Results: In a pooled analysis of double-blind subjects in the ITT population of Study 1 and Study 2, tradipitant demonstrated a clinically meaningful and statistically significant improvement in average nausea at week 4 (-1.15 for tradipitant v. -0.85 for placebo, p=0.0138). Significant improvement was seen in the number of nausea free days (20.96% improvement for tradipitant v. 12.52% for placebo, p=0.0085). Tradipitant also showed improvement in other gastroparesis symptoms as measured by the GCSI total score (-0.99 for tradipitant v. -0.76 for placebo, p=0.0265). Responder rates for PGI-C were 79.3% on tradipitant versus 69% on placebo at week 4 (p value = 0.036). For the Overall Patient Benefit, more patients improved on tradipitant versus placebo with 85.3% v. 71.2% at week 4 (p= 0.002). Sensitivity analysis adjusting for drug compliance confirmed the PK population (tradipitant blood levels ≥ 140ng/mL) in the pooled data set (n=284). In the pooled PK Population, tradipitant significantly improved average nausea at week 4 with -1.38 for tradipitant v. -0.85 for placebo (p=0.0001).
Discussion: Pooling the data provided an opportunity to analyze a much larger data set, increase statistical power, and confirm results and subpopulations from the two separate studies. In the pooled analysis, we observed a clinically meaningful and significant effect on change of nausea severity at week 4. Improvements were also seen across core gastroparesis symptoms and overall measures of gastroparesis.
Disclosures:
Jesse L. Carlin, PhD, Christos Polymeropoulos, MD, Michaela Fisher, BS, Darby Madonick, BA, Caleigh Kupersmith, BA, Paula Moszczynski, MS, Changfu Xiao, PhD, Gunther Birznieks, MS, Mihael H. Polymeropoulos, MD. A0685 - Pooled Efficacy Analysis of Tradipitant in Idiopathic and Diabetic Gastroparesis Study VP-VLY-686-3301 and VP-VLY-686-2301, ACG 2022 Annual Scientific Meeting Abstracts. Charlotte, NC: American College of Gastroenterology.
Jesse L. Carlin, PhD, Christos Polymeropoulos, MD, Michaela Fisher, BS, Darby Madonick, BA, Caleigh Kupersmith, BA, Paula Moszczynski, MS, Changfu Xiao, PhD, Gunther Birznieks, MS, Mihael H. Polymeropoulos, MD
Vanda Pharmaceuticals, Inc., Washington, DC
Introduction: Tradipitant is a novel NK-1 receptor antagonist studied in diabetic and idiopathic gastroparesis for short-term relief of nausea. This report presents the pooled analysis from 2 multicenter, randomized, double-blind, placebo-controlled studies (VP-VLY-686-3301 and VP-VLY-686-2301) assessing the efficacy of tradipitant in relieving symptoms of gastroparesis.
Methods: N=342 idiopathic and diabetic gastroparesis patients with delayed gastric emptying, moderate to severe nausea were included in the pooled ITT (Intent-to-Treat) population. Subjects were randomized to 85mg tradipitant twice a day (n=175) or placebo (n=167) and endpoints were assessed at Week 4. Nausea was assessed with the 5-point Gastroparesis Core Symptom Daily Diary (GCSDD). Overall gastroparesis symptom improvement was evaluated with the Patient Global Impression of Change (PGI-C) and Overall Patient Benefit (OPB) scales. Sensitivity analyses were performed to control for confounders.
Results: In a pooled analysis of double-blind subjects in the ITT population of Study 1 and Study 2, tradipitant demonstrated a clinically meaningful and statistically significant improvement in average nausea at week 4 (-1.15 for tradipitant v. -0.85 for placebo, p=0.0138). Significant improvement was seen in the number of nausea free days (20.96% improvement for tradipitant v. 12.52% for placebo, p=0.0085). Tradipitant also showed improvement in other gastroparesis symptoms as measured by the GCSI total score (-0.99 for tradipitant v. -0.76 for placebo, p=0.0265). Responder rates for PGI-C were 79.3% on tradipitant versus 69% on placebo at week 4 (p value = 0.036). For the Overall Patient Benefit, more patients improved on tradipitant versus placebo with 85.3% v. 71.2% at week 4 (p= 0.002). Sensitivity analysis adjusting for drug compliance confirmed the PK population (tradipitant blood levels ≥ 140ng/mL) in the pooled data set (n=284). In the pooled PK Population, tradipitant significantly improved average nausea at week 4 with -1.38 for tradipitant v. -0.85 for placebo (p=0.0001).
Discussion: Pooling the data provided an opportunity to analyze a much larger data set, increase statistical power, and confirm results and subpopulations from the two separate studies. In the pooled analysis, we observed a clinically meaningful and significant effect on change of nausea severity at week 4. Improvements were also seen across core gastroparesis symptoms and overall measures of gastroparesis.
Disclosures:
Jesse Carlin: Vanda Pharmaceuticals, Inc. – Employee, Stock-publicly held company(excluding mutual/index funds).
Christos Polymeropoulos: Vanda Pharmaceuticals, Inc. – Employee, Stock-publicly held company(excluding mutual/index funds).
Michaela Fisher: Vanda Pharmaceuticals, Inc. – Employee, Stock-publicly held company(excluding mutual/index funds).
Darby Madonick: Vanda Pharmaceuticals, Inc. – Employee, Stock-publicly held company(excluding mutual/index funds).
Caleigh Kupersmith: Vanda Pharmaceuticals, Inc. – Employee, Stock-publicly held company(excluding mutual/index funds).
Paula Moszczynski: Vanda Pharmaceuticals, Inc. – Employee, Stock-publicly held company(excluding mutual/index funds).
Changfu Xiao: Vanda Pharmaceuticals, Inc. – Employee, Stock-publicly held company(excluding mutual/index funds).
Gunther Birznieks: Vanda Pharmaceuticals, Inc. – Employee, Stock-publicly held company(excluding mutual/index funds).
Mihael Polymeropoulos: Vanda Pharmaceuticals, Inc. – Advisory Committee/Board Member, CEO, Stock-publicly held company(excluding mutual/index funds).
Jesse L. Carlin, PhD, Christos Polymeropoulos, MD, Michaela Fisher, BS, Darby Madonick, BA, Caleigh Kupersmith, BA, Paula Moszczynski, MS, Changfu Xiao, PhD, Gunther Birznieks, MS, Mihael H. Polymeropoulos, MD. A0685 - Pooled Efficacy Analysis of Tradipitant in Idiopathic and Diabetic Gastroparesis Study VP-VLY-686-3301 and VP-VLY-686-2301, ACG 2022 Annual Scientific Meeting Abstracts. Charlotte, NC: American College of Gastroenterology.


