Back

Poster Session B - Monday Morning
Category: Liver
B0623 - Drug-Induced Liver Injury Secondary to Gabapentin
Monday, October 24, 2022
10:00 AM – 12:00 PM ET
Location: Crown Ballroom

Has Audio

Michael A. Fayad, DO
Loyola University Medical Center
Maywood, IL
Presenting Author(s)
Michael A. Fayad, DO, Michael Gong, MD, Jamie Berkes, MD
Loyola University Medical Center, Maywood, IL
Introduction: We are reporting a case of drug induced liver injury (DILI) secondary to gabapentin therapy with risk factors for underlying non-alcoholic fatty liver disease (NAFLD).
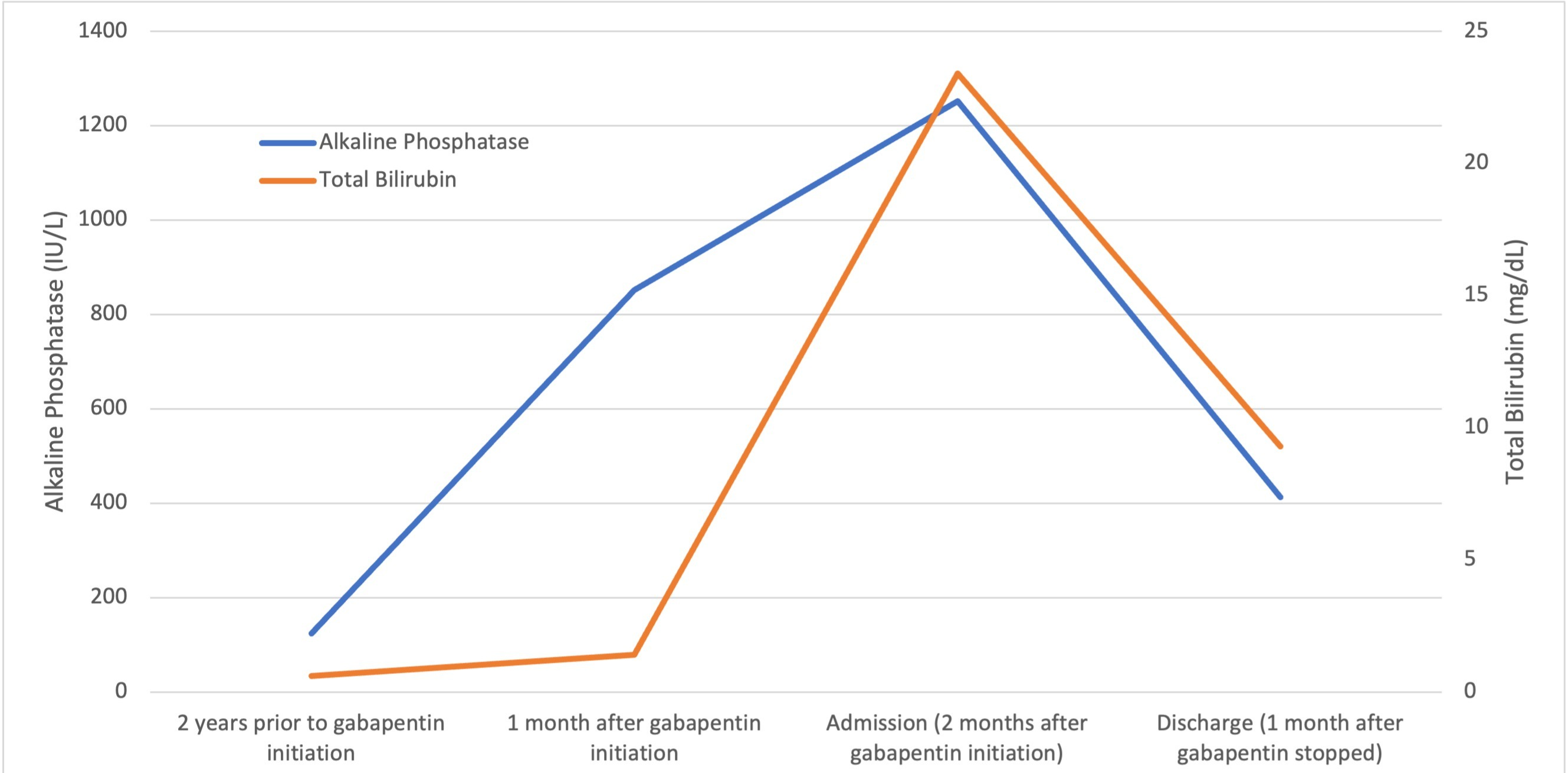
Case Description/Methods: A 56-year-old male with hypertension, hyperlipidemia, diabetes with neuropathy, obesity, and chronic kidney disease stage 3 presented as an outside hospital (OSH) transfer for evaluation of liver transplantation after discovery of acute liver injury. He initially presented to the OSH for jaundice. Admission labs were notable for AST 87, ALT 61, ALP 1252, total bilirubin (Tbili) 23.4, INR 1.1, and creatinine 1.98 (baseline 1.2). Prior lab review showed liver enzymes within normal limits until one month prior to admission, when his ALP was 851. He started taking gabapentin, without introduction of any other medications, one month prior to the initial rise in ALP . Evaluation for viral, inherited, and metabolic causes of liver disease were negative. Liver biopsy showed multifocal hepatocyte cholestasis predominantly involving zone 3 with associated hepatocyte feathery degeneration and neutrophil and lymphocyte infiltration. There was patchy portal edema, mild portal inflammation with neutrophil and lymphocyte infiltrates, and bile duct injury. Trichrome stain highlighted periportal and focal bridging fibrosis appearing to be unrelated to cholestasis, most likely due to underlying NAFLD. The leading differential for cholestasis was drug induced liver injury (DILI) versus biliary obstruction. Magnetic resonance cholangiopancreatography showed no biliary abnormalities. After gabapentin was discontinued, liver enzymes began to downtrend with discharge values of AST 16, ALT 35, ALP 413, Tbili 9.3 and INR 1.1.
Discussion: Gabapentin induced liver injury is rare with few reported cases, many of which did not exclude other etiologies. In this case, the key elements of diagnosing DILI were met including gabapentin initiation closely preceding liver injury, other etiologies excluded, and discontinuation of gabapentin leading to improvement. The severity of this patient’s DILI and general recommendation to avoid future exposure precluded him from being rechallenged with gabapentin. Given the extensive use of gabapentin in medical practice, this case represents an uncommon, but severe, complication of its use. This is also an example of DILI with suspected underlying NAFLD. While NAFLD has not been shown to predispose to DILI, it is suspected to be associated with more serious liver injury in patients who develop DILI.
Disclosures:
Michael A. Fayad, DO, Michael Gong, MD, Jamie Berkes, MD. B0623 - Drug-Induced Liver Injury Secondary to Gabapentin, ACG 2022 Annual Scientific Meeting Abstracts. Charlotte, NC: American College of Gastroenterology.
Loyola University Medical Center, Maywood, IL
Introduction: We are reporting a case of drug induced liver injury (DILI) secondary to gabapentin therapy with risk factors for underlying non-alcoholic fatty liver disease (NAFLD).
Case Description/Methods: A 56-year-old male with hypertension, hyperlipidemia, diabetes with neuropathy, obesity, and chronic kidney disease stage 3 presented as an outside hospital (OSH) transfer for evaluation of liver transplantation after discovery of acute liver injury. He initially presented to the OSH for jaundice. Admission labs were notable for AST 87, ALT 61, ALP 1252, total bilirubin (Tbili) 23.4, INR 1.1, and creatinine 1.98 (baseline 1.2). Prior lab review showed liver enzymes within normal limits until one month prior to admission, when his ALP was 851. He started taking gabapentin, without introduction of any other medications, one month prior to the initial rise in ALP . Evaluation for viral, inherited, and metabolic causes of liver disease were negative. Liver biopsy showed multifocal hepatocyte cholestasis predominantly involving zone 3 with associated hepatocyte feathery degeneration and neutrophil and lymphocyte infiltration. There was patchy portal edema, mild portal inflammation with neutrophil and lymphocyte infiltrates, and bile duct injury. Trichrome stain highlighted periportal and focal bridging fibrosis appearing to be unrelated to cholestasis, most likely due to underlying NAFLD. The leading differential for cholestasis was drug induced liver injury (DILI) versus biliary obstruction. Magnetic resonance cholangiopancreatography showed no biliary abnormalities. After gabapentin was discontinued, liver enzymes began to downtrend with discharge values of AST 16, ALT 35, ALP 413, Tbili 9.3 and INR 1.1.
Discussion: Gabapentin induced liver injury is rare with few reported cases, many of which did not exclude other etiologies. In this case, the key elements of diagnosing DILI were met including gabapentin initiation closely preceding liver injury, other etiologies excluded, and discontinuation of gabapentin leading to improvement. The severity of this patient’s DILI and general recommendation to avoid future exposure precluded him from being rechallenged with gabapentin. Given the extensive use of gabapentin in medical practice, this case represents an uncommon, but severe, complication of its use. This is also an example of DILI with suspected underlying NAFLD. While NAFLD has not been shown to predispose to DILI, it is suspected to be associated with more serious liver injury in patients who develop DILI.

Figure: The figure above depicts the patient’s trend in alkaline phosphatase and total bilirubin starting at a baseline obtained 2 years prior to initiation of gabapentin through to his discharge (one month after gabapentin was stopped). The x-axis show chronology of gabapentin administration. The left y-axis shows alkaline phosphatase levels in IU/L. The right y-axis shows total bilirubin levels in mg/dL.
Disclosures:
Michael Fayad indicated no relevant financial relationships.
Michael Gong indicated no relevant financial relationships.
Jamie Berkes indicated no relevant financial relationships.
Michael A. Fayad, DO, Michael Gong, MD, Jamie Berkes, MD. B0623 - Drug-Induced Liver Injury Secondary to Gabapentin, ACG 2022 Annual Scientific Meeting Abstracts. Charlotte, NC: American College of Gastroenterology.
