Back


Poster Session B - Monday Morning
Category: Small Intestine
B0635 - Evaluation of the Pharmacokinetics of PGN-OB1 Following Oral Administration of an Oral Biotherapeutics Delivery System (OBDS) in Yucatan Swine
Monday, October 24, 2022
10:00 AM – 12:00 PM ET
Location: Crown Ballroom

Has Audio
- SL
Shaoying N. Lee, PhD
Biora Therapeutics
San Diego, CA
Presenting Author(s)
Shaoying N. Lee, PhD1, Cheryl Stork, PhD1, Jeffrey A. Smith, PhD2, Bryan Smith, MS1, Nelson Quintana, BA1, Chris Wahl, MD, PhD1, Sharat Singh, PhD1
1Biora Therapeutics, San Diego, CA; 2Biora Therapeutics, Irving, TX
Introduction: Biologics/peptides/nucleic acids are highly effective drugs; however, oral delivery of these therapeutics has proved to be difficult due to the harsh conditions of the upper gastrointestinal tract (GIT) and the poor absorption rate in the small intestinal mucosa. We aimed to develop an oral biotherapeutic delivery system (OBDS) that protects these biomolecules from degradation in the upper GIT and increases oral bioavailability via needless injection in the submucosal space of the small intestine. We describe the development of models to assess the functional capability of the OBDS capsule. A Yucatan minipig model was chosen to better represent the pharmacokinetic properties of submucosal injection in humans and evaluate the injection efficiency of OBDS in vivo.
Methods: Due to prolonged and variable gastric residence times in the swine model, the semi-autonomous OBDS capsules containing a variant of adalimumab (PGN-001), PGN-OB1, were administrated by intraduodenal endoscopic placement (ID) and released into the proximal small intestines of the female Yucatan swine. Blood samples post-ID dosing were collected to evaluate the injection efficiency of the OBDS compared with the IV control group.
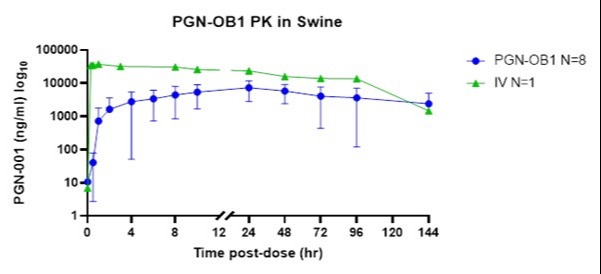
Results: All OBDS capsules were successfully advanced through the pyloric sphincter via endoscopic placement, without early deployment, and released in the proximal duodenum to naturally transit and deploy in vivo. Out of 13 animals dosed, 8 animals showed detectable drug levels (Figure 1), and an oral bioavailability average of 22% or 25% (range from 7-55%) excluding an animal showing a late deployment at 72hr post-dose. The variability in the swine model is believed to be due to physiologic variability that may not directly translate to humans including variability in small intestinal transit time, and more frequent and larger, gas and fluid pockets in the swine.
Discussion: Here we have provided proof-of-concept of a semi-autonomous OBDS device that can achieve as high as 55% bioavailability of a variant of adalimumab that is a magnitude higher than current oral protein or peptide delivery technology in the market and at levels much closer to the subcutaneous route of administration estimated in human trials. The OBDS capsules could potentially provide a better alternative for the non-invasive route of administration with better patient compliance.
Disclosures:
Shaoying N. Lee, PhD1, Cheryl Stork, PhD1, Jeffrey A. Smith, PhD2, Bryan Smith, MS1, Nelson Quintana, BA1, Chris Wahl, MD, PhD1, Sharat Singh, PhD1. B0635 - Evaluation of the Pharmacokinetics of PGN-OB1 Following Oral Administration of an Oral Biotherapeutics Delivery System (OBDS) in Yucatan Swine, ACG 2022 Annual Scientific Meeting Abstracts. Charlotte, NC: American College of Gastroenterology.
1Biora Therapeutics, San Diego, CA; 2Biora Therapeutics, Irving, TX
Introduction: Biologics/peptides/nucleic acids are highly effective drugs; however, oral delivery of these therapeutics has proved to be difficult due to the harsh conditions of the upper gastrointestinal tract (GIT) and the poor absorption rate in the small intestinal mucosa. We aimed to develop an oral biotherapeutic delivery system (OBDS) that protects these biomolecules from degradation in the upper GIT and increases oral bioavailability via needless injection in the submucosal space of the small intestine. We describe the development of models to assess the functional capability of the OBDS capsule. A Yucatan minipig model was chosen to better represent the pharmacokinetic properties of submucosal injection in humans and evaluate the injection efficiency of OBDS in vivo.
Methods: Due to prolonged and variable gastric residence times in the swine model, the semi-autonomous OBDS capsules containing a variant of adalimumab (PGN-001), PGN-OB1, were administrated by intraduodenal endoscopic placement (ID) and released into the proximal small intestines of the female Yucatan swine. Blood samples post-ID dosing were collected to evaluate the injection efficiency of the OBDS compared with the IV control group.
Results: All OBDS capsules were successfully advanced through the pyloric sphincter via endoscopic placement, without early deployment, and released in the proximal duodenum to naturally transit and deploy in vivo. Out of 13 animals dosed, 8 animals showed detectable drug levels (Figure 1), and an oral bioavailability average of 22% or 25% (range from 7-55%) excluding an animal showing a late deployment at 72hr post-dose. The variability in the swine model is believed to be due to physiologic variability that may not directly translate to humans including variability in small intestinal transit time, and more frequent and larger, gas and fluid pockets in the swine.
Discussion: Here we have provided proof-of-concept of a semi-autonomous OBDS device that can achieve as high as 55% bioavailability of a variant of adalimumab that is a magnitude higher than current oral protein or peptide delivery technology in the market and at levels much closer to the subcutaneous route of administration estimated in human trials. The OBDS capsules could potentially provide a better alternative for the non-invasive route of administration with better patient compliance.

Figure: Figure 1. Plasma concentration of PGN-001 treated with ID and IV over time
Disclosures:
Shaoying Lee: Biora Therapeutics – Employee. crohn's and colitis foundation – Grant/Research Support.
Cheryl Stork: Biora Therapeutics – Employee.
Jeffrey Smith: Biora Therapeutics – Employee.
Bryan Smith: Biora Therapeutics – Employee.
Nelson Quintana: Biora Therapeutics – Employee.
Chris Wahl: Biora Therapeutics – Employee.
Sharat Singh: Bioratherapeutics – Employee.
Shaoying N. Lee, PhD1, Cheryl Stork, PhD1, Jeffrey A. Smith, PhD2, Bryan Smith, MS1, Nelson Quintana, BA1, Chris Wahl, MD, PhD1, Sharat Singh, PhD1. B0635 - Evaluation of the Pharmacokinetics of PGN-OB1 Following Oral Administration of an Oral Biotherapeutics Delivery System (OBDS) in Yucatan Swine, ACG 2022 Annual Scientific Meeting Abstracts. Charlotte, NC: American College of Gastroenterology.
