Back

Poster Session C - Monday Afternoon
Category: Functional Bowel Disease
C0268 - Efficacy of Tenapanor in Improving IBS-C Abdominal Symptoms: A Post Hoc Analysis of Multi-Item Abdominal Score From the 26-Week Phase 3 T3MPO-2 Study
Monday, October 24, 2022
3:00 PM – 5:00 PM ET
Location: Crown Ballroom

Has Audio
- AL
Anthony Lembo, MD, FACG
Beth Israel Deaconess Medical Center and Harvard Medical School
Boston, Massachusetts
Presenting Author(s)
Anthony Lembo, MD, FACG1, William D. Chey, MD2, Susan Edelstein, PhD3, Yang Yang, PhD3, David M. Spiegel, MD3, David P. Rosenbaum, PhD3
1Beth Israel Deaconess Medical Center and Harvard Medical School, Boston, MA; 2Michigan Medicine, Ann Arbor, MI; 3Ardelyx, Inc., Waltham, MA
Introduction: Tenapanor is a minimally absorbed, small-molecule inhibitor of intestinal sodium/hydrogen exchanger 3 (NHE3), approved for the treatment of irritable bowel syndrome with constipation (IBS-C). Preclinical studies demonstrated that tenapanor reduced intestinal permeability, inhibited TRPV1 signaling, and was associated with reduced visceral hypersensitivity and abdominal pain. Here we investigate the effects of tenapanor on multi-item abdominal score using data from T3MPO-2 (NCT02686138), a long-term phase 3 study of tenapanor.
Methods: Patients with IBS-C with < 3 weekly complete spontaneous bowel movements and weekly abdominal pain score ≥3 (0-10 scale) during a 2-week screening period were randomized to tenapanor 50 mg or placebo twice a day in a 26-week randomized treatment period (RTP). Patients rated 5 abdominal symptoms on an 11-point scale (0=no symptom to 10=worst possible symptom) using an eDiary.
The abdominal score 3 (AS3) is the mean of weekly scores for abdominal pain, discomfort, and bloating. The abdominal score 5 (AS5) is the mean of weekly scores for abdominal pain, discomfort, bloating, fullness, and cramping. The overall change from baseline (CFB) in the 26-week RTP and week 26 CFB in AS3 and AS5 were compared between arms using mixed-effects models with repeated measures. The cumulative distribution of CFB in AS3 or AS5 at week 26 was compared along with the Wilcoxon rank sum test. The 13/26-week AS3 or AS5 response, defined as achieving a reduction of ≥2 points in AS3 or AS5 for ≥13 weeks of the 26-week RTP, was compared using the Pearson’s chi-square test.
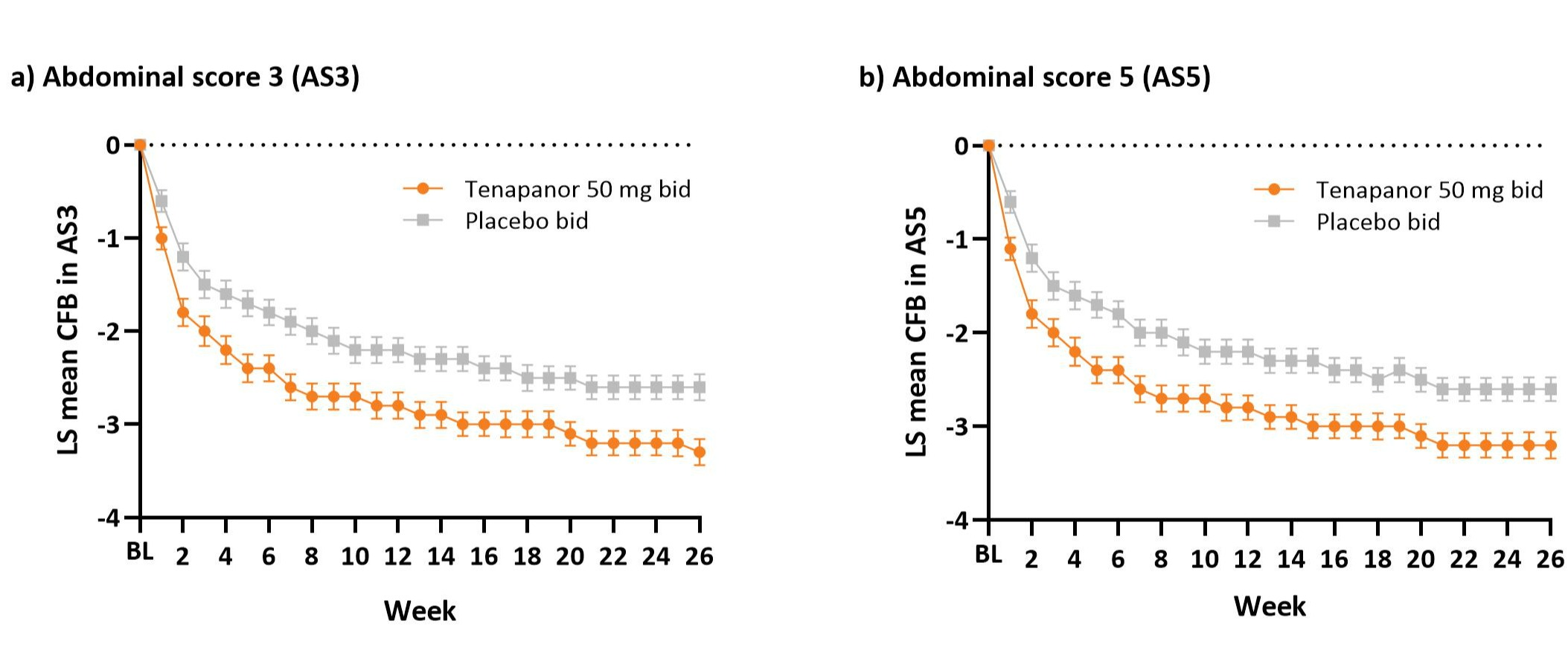
Results: T3MPO-2 randomized 620 patients. In the intent-to-treat analysis set (tenapanor, n=293; placebo, n=300), the tenapanor arm had greater mean reduction in AS3 than placebo over the 26-week RTP (Figure a; −2.74 vs −2.15, P=0.0001) and in week 26 (−3.27 vs −2.60, P=0.0007). At week 26, cumulative distribution of CFB significantly favored tenapanor over placebo (P=0.0094). The tenapanor arm also had a higher 13/26-week AS3 response rate than placebo (46.4% vs 35.7%, P=0.0078). For AS5, mean CFB (Figure b; P=0.0001), distribution of CFB at week 26 (P=0.0121), and 13/26-week response rate (P=0.0015) were similarly improved with tenapanor.
Discussion: Few treatments for IBS-C improve abdominal pain, discomfort, or bloating. This post hoc analysis demonstrates that tenapanor significantly improves IBS-C–associated abdominal symptoms with an early onset of action that is sustained throughout the treatment period.
Disclosures:
Anthony Lembo, MD, FACG1, William D. Chey, MD2, Susan Edelstein, PhD3, Yang Yang, PhD3, David M. Spiegel, MD3, David P. Rosenbaum, PhD3. C0268 - Efficacy of Tenapanor in Improving IBS-C Abdominal Symptoms: A Post Hoc Analysis of Multi-Item Abdominal Score From the 26-Week Phase 3 T3MPO-2 Study, ACG 2022 Annual Scientific Meeting Abstracts. Charlotte, NC: American College of Gastroenterology.
1Beth Israel Deaconess Medical Center and Harvard Medical School, Boston, MA; 2Michigan Medicine, Ann Arbor, MI; 3Ardelyx, Inc., Waltham, MA
Introduction: Tenapanor is a minimally absorbed, small-molecule inhibitor of intestinal sodium/hydrogen exchanger 3 (NHE3), approved for the treatment of irritable bowel syndrome with constipation (IBS-C). Preclinical studies demonstrated that tenapanor reduced intestinal permeability, inhibited TRPV1 signaling, and was associated with reduced visceral hypersensitivity and abdominal pain. Here we investigate the effects of tenapanor on multi-item abdominal score using data from T3MPO-2 (NCT02686138), a long-term phase 3 study of tenapanor.
Methods: Patients with IBS-C with < 3 weekly complete spontaneous bowel movements and weekly abdominal pain score ≥3 (0-10 scale) during a 2-week screening period were randomized to tenapanor 50 mg or placebo twice a day in a 26-week randomized treatment period (RTP). Patients rated 5 abdominal symptoms on an 11-point scale (0=no symptom to 10=worst possible symptom) using an eDiary.
The abdominal score 3 (AS3) is the mean of weekly scores for abdominal pain, discomfort, and bloating. The abdominal score 5 (AS5) is the mean of weekly scores for abdominal pain, discomfort, bloating, fullness, and cramping. The overall change from baseline (CFB) in the 26-week RTP and week 26 CFB in AS3 and AS5 were compared between arms using mixed-effects models with repeated measures. The cumulative distribution of CFB in AS3 or AS5 at week 26 was compared along with the Wilcoxon rank sum test. The 13/26-week AS3 or AS5 response, defined as achieving a reduction of ≥2 points in AS3 or AS5 for ≥13 weeks of the 26-week RTP, was compared using the Pearson’s chi-square test.
Results: T3MPO-2 randomized 620 patients. In the intent-to-treat analysis set (tenapanor, n=293; placebo, n=300), the tenapanor arm had greater mean reduction in AS3 than placebo over the 26-week RTP (Figure a; −2.74 vs −2.15, P=0.0001) and in week 26 (−3.27 vs −2.60, P=0.0007). At week 26, cumulative distribution of CFB significantly favored tenapanor over placebo (P=0.0094). The tenapanor arm also had a higher 13/26-week AS3 response rate than placebo (46.4% vs 35.7%, P=0.0078). For AS5, mean CFB (Figure b; P=0.0001), distribution of CFB at week 26 (P=0.0121), and 13/26-week response rate (P=0.0015) were similarly improved with tenapanor.
Discussion: Few treatments for IBS-C improve abdominal pain, discomfort, or bloating. This post hoc analysis demonstrates that tenapanor significantly improves IBS-C–associated abdominal symptoms with an early onset of action that is sustained throughout the treatment period.

Figure: Figure. Treatment with tenapanor resulted in a greater and sustained reduction from baseline in combined abdominal symptom scores over the 26-week treatment period vs placebo.
Error bars represent standard error.
AS3, abdominal score 3; AS5, abdominal score 5; bid, twice a day; BL, baseline; CFB, change from baseline; LS, least squares.
Error bars represent standard error.
AS3, abdominal score 3; AS5, abdominal score 5; bid, twice a day; BL, baseline; CFB, change from baseline; LS, least squares.
Disclosures:
Anthony Lembo: Alkermes – Consultant. Arena Pharmaceuticals, Inc. – Consultant. Bayer – Consultant. Bristol-Myers Squibb – Stock Options. Gemelli Biotech – Consultant. Ironwood Pharmaceuticals, Inc. – Consultant. Johnson & Johnson – Stock Options. OrphoMed, Inc. – Consultant. Salix Pharmaceuticals, Inc. – Consultant. Shire, a Takeda company – Consultant. Takeda Pharmaceuticals – Consultant. Vibrant – Advisor or Review Panel Member. Vibrant Pharma Inc. – Consultant.
William Chey: Abbvie – Consultant. Allakos – Consultant. Alnylam – Consultant. Arena – Consultant. Biomerica – Consultant. Commonwealth Diagnostics International – Consultant. Gemelli – Consultant. GI OnDEMAND – Stock Options. Ironwood – Consultant. Isothrive – Consultant. Isothrive – Stock Options. Modify Health – Stock Options. Nestle – Consultant. Phathom – Consultant. Progenity – Consultant. QOL Medical – Consultant, Grant/Research Support. Redhill – Consultant. Salix – Consultant, Grant/Research Support. Urovant – Consultant. Vibrant – Consultant.
Susan Edelstein: Ardelyx, Inc. – Employee.
Yang Yang: Ardelyx, Inc. – Employee.
David Spiegel: Ardelyx, Inc. – Employee.
David Rosenbaum: Ardelyx, Inc. – Employee.
Anthony Lembo, MD, FACG1, William D. Chey, MD2, Susan Edelstein, PhD3, Yang Yang, PhD3, David M. Spiegel, MD3, David P. Rosenbaum, PhD3. C0268 - Efficacy of Tenapanor in Improving IBS-C Abdominal Symptoms: A Post Hoc Analysis of Multi-Item Abdominal Score From the 26-Week Phase 3 T3MPO-2 Study, ACG 2022 Annual Scientific Meeting Abstracts. Charlotte, NC: American College of Gastroenterology.
