Poster Session C - Monday Afternoon
Category: Functional Bowel Disease
C0277 - Vibrating Capsule Is Efficacious in Patients With Severe Chronic Idiopathic Constipation (CIC)

Satish Rao, MD
Augusta University
Augusta, Georgia
Presenting Author(s)
1Augusta University, Augusta, GA; 2Michigan Medicine, Ann Arbor, MI; 3Houston Methodist Hospital, Houston, TX; 4Beth Israel Deaconess Medical Center and Harvard Medical School, Boston, MA; 5Northwestern Medical Group, Chicago, IL; 6Cedars-Sinai Medical Center, Los Angeles, CA; 7Cornell University, New York, NY
Introduction: Approximately 50 million Americans report constipation, of whom 28% have moderately severe illness (Am J Gastro 2020;115:895-905). The effectiveness of current therapies for severe chronic idiopathic constipation (CIC) is unknown. In a recent Phase III trial, a vibrating capsule (VC) proved superior to placebo in improving bowel and abdominal symptoms in patients with CIC. Aim: To determine the efficacy and safety of VC in patients with severe CIC.
Methods: We performed a post-hoc analysis of CIC patients (Rome III) who were enrolled in an 8-week phase 3, multicenter, double-blind trial, and randomly received one VC (Vibrant, Yokneam, Israel) or placebo orally for 5 days/week. Severe CIC was defined as patients who reported 0 complete spontaneous bowel movements (CSBM) during a 2-week baseline period on a daily electronic stool diary. Patients with history of dysphagia or bowel obstruction were excluded. Primary outcome measures were percentage of subjects with an increase of one (CSBM1), two (CSBM2), or three (CSBM3) CSBM/week, during at least 6 of 8 treatment weeks compared to baseline. Secondary outcomes and safety were also assessed.
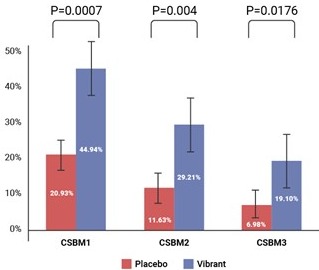
Results: 312 CIC patients were enrolled of whom 56% (VC n=89, placebo n=86) had severe CIC. There were significantly greater CSBM1 (p=0.0007), CSBM2 (p=0.0040), and CSBM3 (p=0.0176), responders in the VC group compared to placebo (Fig). The straining effort (p=0.0027) and stool consistency (p=< .0001) also improved significantly in the VC group compared to placebo. Also, QOL significantly improved in the VC group (p=< .0001) compared to placebo. The treatment was generally safe, without severe adverse events or diarrhea. The most common AEs in the group were a sensation of mild vibration (11.2%) and abdominal discomfort (2.25%), table 1.
Discussion: In individuals with severe CIC, VC significantly improved bowel and abdominal symptoms and QOL compared to placebo. The VC was safe and well tolerated. Vibrating Capsule is a first in class, novel, non-pharmacological treatment that is efficacious and safe in patients with severe chronic constipation.
Adverse event | Vibrating Capsule, Mode 1 (n=89) No. of patients (%) | Placebo (n=86) No. of patients (%) |
Adverse events during treatment (combined safety populations including interim analysis groups).* | ||
Any event | 23 (25.84) | 15 (17.4) |
Sensation of vibration** | 10 (11.24) | |
Headache | 1 (1.12) | 1 (1.16) |
Urinary tract infection | 1 (1.12) | 1 (1.16) |
Abdominal pain | 2 (2.23) | |
Abdominal discomfort | 2 (2.25) | |
Vomiting | 2 (2.25) | 1 (1.16) |
Nausea | 3 (3.37) | 1 (1.16) |
Abdominal distention | 2 (2.23) | |
Diarrhea | 2 (2.25) | |
Covid-19 | 1 (1.12) | 1 (1.16) |
Nasopharyngitis/Bronchitis | 2 (2.25) | |
Musculoskeletal | 1 (1.16) | |
*Data shown for adverse events in at least 1% of the subjects ** Sensation of vibration means: ``I think I felt vibration`` | ||
Disclosures:
Satish Rao, MD1, William D. Chey, MD2, Eamonn M. Quigley, MD, MACG3, Anthony Lembo, MD, FACG4, Darren Brenner, MD5, Brennan Spiegel, MD6, Christine Frissora, MD7. C0277 - Vibrating Capsule Is Efficacious in Patients With Severe Chronic Idiopathic Constipation (CIC), ACG 2022 Annual Scientific Meeting Abstracts. Charlotte, NC: American College of Gastroenterology.
