Back


Poster Session C - Monday Afternoon
Category: IBD
C0368 - Comparative Effectiveness of Vedolizumab versus Ustekinumab in Anti-TNF Experienced Patients With Crohn’s Disease
Monday, October 24, 2022
3:00 PM – 5:00 PM ET
Location: Crown Ballroom

Has Audio

Michael Kappelman, MD
University of North Carolina at Chapel Hill
Chapel Hill, NC
Presenting Author(s)
Michael Kappelman, MD, MPH1, James D. Lewis, MD, MSCE2, Xian Zhang, PhD3, Feng-Chang Lin, PhD4, Laura Weisbein, PhD1, Wenli Chen, MS, MA5, Jessica L. Burris, MD6, Jennifer E. Dorand, PhD7, Lauren E. Parlett, BS, PhD8, Kevin Haynes, PharmD, MSc(Epi)9, Vinit Nair, MS, RPH10, Alan F. Kaul, MS, PharmD, MBA11, Angela Dobes, MPH12, Millie Long, MD, MPH, FACG1
1UNC Chapel Hill, Chapel Hill, NC; 2University of Pennsylvania, Philadelphia, PA; 3University of North Carolina at Chapel Hill School of Medicine, Chapel Hill, NC; 4University of North Carolina at Chapel Hill, Chapel Hill, NC; 5UNC CGIBD, Chapel Hill, NC; 6Yale University School of Medicine, West Haven, CT; 7Crohn’s & Colitis Foundation, Somerset, NJ; 8HealthCore, Inc., Wilmington, DE; 9Janssen Research & Development, Wayne, PA; 10Sunrise, FL; 11Medical Outcomes Management, Inc., Sharon, MA; 12Crohn's & Colitis Foundation, New York, NY
Introduction: Primary and secondary non-response to anti-TNF therapy is common in patients with Crohn’s disease (CD), yet there is a paucity of research comparing vedolizumab and ustekinumab as subsequent therapy. Prior studies, limited to tertiary care centers in Europe, have yielded conflicting results and did not include reported outcomes (PROs). We sought to compare the effectiveness of vedolizumab and ustekinumab in anti-TNF experienced patients with CD, focusing on patient-prioritized PROs.
Methods: We utilized the IBD Partners internet-based research infrastructure to conduct a prospective, direct-to-patient cohort study in a geographically diverse U.S. population. Within IBD Partners, participants report disease characteristics, current and prior treatments, and PROs every 6 months. For this analysis, we identified anti-TNF experienced patients with CD initiating vedolizumab or ustekinumab and analyzed PROs reported approximately 6 months later (minimum 4 months, maximum 10 months). Co-primary outcomes were Patient Reported Outcome Measurement Information System (PROMIS) domains of Fatigue and Pain Interference. Secondary outcomes included patient-reported short Crohn’s Disease Activity Index (sCDAI) and treatment persistence and corticosteroid use at the time of follow-up. Inverse probability of treatment weighting (IPTW) was used to control for potential confounders and incorporated into linear and logistic regression models for linear and categorical outcomes, respectively.
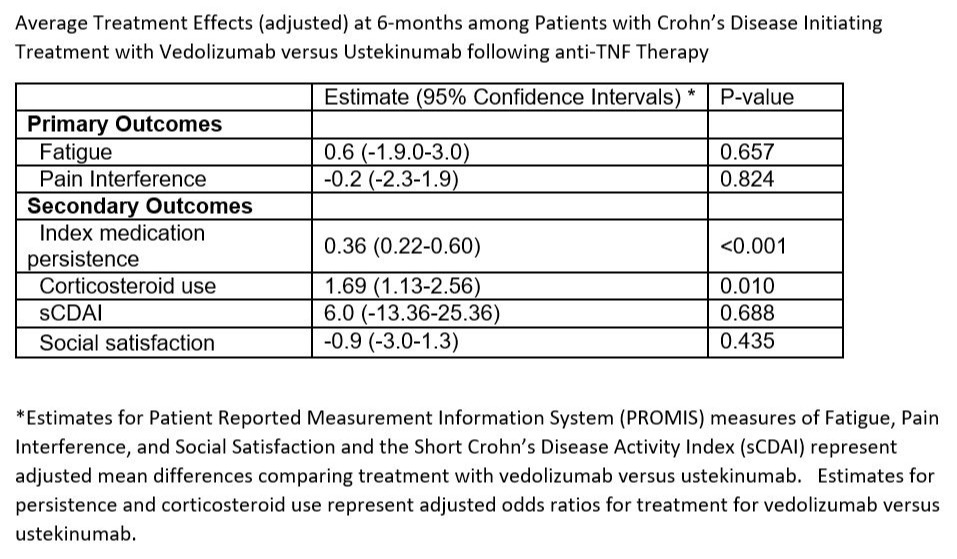
Results: Overall, 141 vedolizumab and 219 ustekinumab initiators were included in our analysis. After adjustment, we found no differences in our primary outcomes of Pain Interference or Fatigue or the secondary outcome of sCDAI between initiators of vedolizumab versus ustekinumab (Table). However, vedolizumab was associated with lower treatment persistence (OR 0.36, 95% CI 0.22-0.60) and higher corticosteroid use at follow-up assessment (OR 1.69, 95% CI 1.13-2.56).
Discussion: Vedolizumab and ustekinumab are similarly effective at 6 months, as measured by a broad panel of patient-centered outcomes; although we observed higher treatment persistence and lower corticosteroid use among ustekinumab users. Hence, factors such as patient preference, route of administration, cost, and data regarding other outcomes must be considered when making decisions about subsequent treatment options.
Disclosures:
Michael Kappelman, MD, MPH1, James D. Lewis, MD, MSCE2, Xian Zhang, PhD3, Feng-Chang Lin, PhD4, Laura Weisbein, PhD1, Wenli Chen, MS, MA5, Jessica L. Burris, MD6, Jennifer E. Dorand, PhD7, Lauren E. Parlett, BS, PhD8, Kevin Haynes, PharmD, MSc(Epi)9, Vinit Nair, MS, RPH10, Alan F. Kaul, MS, PharmD, MBA11, Angela Dobes, MPH12, Millie Long, MD, MPH, FACG1. C0368 - Comparative Effectiveness of Vedolizumab versus Ustekinumab in Anti-TNF Experienced Patients With Crohn’s Disease, ACG 2022 Annual Scientific Meeting Abstracts. Charlotte, NC: American College of Gastroenterology.
1UNC Chapel Hill, Chapel Hill, NC; 2University of Pennsylvania, Philadelphia, PA; 3University of North Carolina at Chapel Hill School of Medicine, Chapel Hill, NC; 4University of North Carolina at Chapel Hill, Chapel Hill, NC; 5UNC CGIBD, Chapel Hill, NC; 6Yale University School of Medicine, West Haven, CT; 7Crohn’s & Colitis Foundation, Somerset, NJ; 8HealthCore, Inc., Wilmington, DE; 9Janssen Research & Development, Wayne, PA; 10Sunrise, FL; 11Medical Outcomes Management, Inc., Sharon, MA; 12Crohn's & Colitis Foundation, New York, NY
Introduction: Primary and secondary non-response to anti-TNF therapy is common in patients with Crohn’s disease (CD), yet there is a paucity of research comparing vedolizumab and ustekinumab as subsequent therapy. Prior studies, limited to tertiary care centers in Europe, have yielded conflicting results and did not include reported outcomes (PROs). We sought to compare the effectiveness of vedolizumab and ustekinumab in anti-TNF experienced patients with CD, focusing on patient-prioritized PROs.
Methods: We utilized the IBD Partners internet-based research infrastructure to conduct a prospective, direct-to-patient cohort study in a geographically diverse U.S. population. Within IBD Partners, participants report disease characteristics, current and prior treatments, and PROs every 6 months. For this analysis, we identified anti-TNF experienced patients with CD initiating vedolizumab or ustekinumab and analyzed PROs reported approximately 6 months later (minimum 4 months, maximum 10 months). Co-primary outcomes were Patient Reported Outcome Measurement Information System (PROMIS) domains of Fatigue and Pain Interference. Secondary outcomes included patient-reported short Crohn’s Disease Activity Index (sCDAI) and treatment persistence and corticosteroid use at the time of follow-up. Inverse probability of treatment weighting (IPTW) was used to control for potential confounders and incorporated into linear and logistic regression models for linear and categorical outcomes, respectively.
Results: Overall, 141 vedolizumab and 219 ustekinumab initiators were included in our analysis. After adjustment, we found no differences in our primary outcomes of Pain Interference or Fatigue or the secondary outcome of sCDAI between initiators of vedolizumab versus ustekinumab (Table). However, vedolizumab was associated with lower treatment persistence (OR 0.36, 95% CI 0.22-0.60) and higher corticosteroid use at follow-up assessment (OR 1.69, 95% CI 1.13-2.56).
Discussion: Vedolizumab and ustekinumab are similarly effective at 6 months, as measured by a broad panel of patient-centered outcomes; although we observed higher treatment persistence and lower corticosteroid use among ustekinumab users. Hence, factors such as patient preference, route of administration, cost, and data regarding other outcomes must be considered when making decisions about subsequent treatment options.

Figure: Average Treatment Effects (adjusted) at 6-months among Patients with Crohn’s Disease Initiating Treatment with Vedolizumab versus Ustekinumab following anti-TNF Therapy
Disclosures:
Michael Kappelman: Abbvie – Consultant. Eli Lilly – Consultant. Janssen – Consultant, Stock-publicly held company(excluding mutual/index funds). Pfizer – Consultant. Takeda – Consultant.
James Lewis: AbbVie – Grant/Research Support. Amgen – Advisor or Review Panel Member. Arena Pharmaceuticals – Advisor or Review Panel Member. BMS – Consultant. Bridge Biotherapeutics – Consultant. Dark Canyon Laboratories – Consultant, Stock-privately held company. Entasis Therapeutics – Consultant. Gilead – Advisor or Review Panel Member. Janssen Pharmaceuticals – Consultant, Grant/Research Support. Merck – Consultant. Multiple manufactures of generic ranitidine – Expert witness. Nestle Health Science – Consultant, Grant/Research Support. Pfizer – Advisor or Review Panel Member. Protagonist Therapeutics – Advisor or Review Panel Member. Takeda – Grant/Research Support.
Xian Zhang indicated no relevant financial relationships.
Feng-Chang Lin indicated no relevant financial relationships.
Laura Weisbein indicated no relevant financial relationships.
Wenli Chen indicated no relevant financial relationships.
Jessica Burris indicated no relevant financial relationships.
Jennifer Dorand: Crohn’s & Colitis Foundation – Advisory Committee/Board Member. East Orange VA Medical Center – Employee. Pfizer Inc. – Stock-publicly held company(excluding mutual/index funds). Princeton ProCure Management LLC – Employee. Regeneron Pharmaceuticals – Stock-publicly held company(excluding mutual/index funds).
Lauren Parlett: Sanofi – Grant/Research Support.
Kevin Haynes: Anthem, Inc. – Employee, Stock Options, Stock-publicly held company(excluding mutual/index funds). Janssen Research & Development – Employee.
Vinit Nair indicated no relevant financial relationships.
Alan Kaul indicated no relevant financial relationships.
Angela Dobes indicated no relevant financial relationships.
Millie Long: AbbVie – Consultant. Bristol Myers Squibb – Consultant. Calibr – Consultant. Eli Lilly – Consultant. Genentech – Consultant. Janssen – Consultant. Pfizer – Consultant, Grant/Research Support. Prometheus – Consultant. Roche – Consultant. Salix – Consultant. Takeda – Consultant, Grant/Research Support. Target RWE – Consultant. Theravance – Consultant.
Michael Kappelman, MD, MPH1, James D. Lewis, MD, MSCE2, Xian Zhang, PhD3, Feng-Chang Lin, PhD4, Laura Weisbein, PhD1, Wenli Chen, MS, MA5, Jessica L. Burris, MD6, Jennifer E. Dorand, PhD7, Lauren E. Parlett, BS, PhD8, Kevin Haynes, PharmD, MSc(Epi)9, Vinit Nair, MS, RPH10, Alan F. Kaul, MS, PharmD, MBA11, Angela Dobes, MPH12, Millie Long, MD, MPH, FACG1. C0368 - Comparative Effectiveness of Vedolizumab versus Ustekinumab in Anti-TNF Experienced Patients With Crohn’s Disease, ACG 2022 Annual Scientific Meeting Abstracts. Charlotte, NC: American College of Gastroenterology.
