Back


Poster Session C - Monday Afternoon
Category: IBD
C0384 - Tofacitinib-Associated Adverse Vascular Events Reported to the Federal Adverse Event Reporting System (FAERS) Database
Monday, October 24, 2022
3:00 PM – 5:00 PM ET
Location: Crown Ballroom

Has Audio

Sam D. Papasotiriou, BS
Advocate Lutheran General
Park Ridge, IL
Presenting Author(s)
Sam D. Papasotiriou, BS1, Ryan Meader, DO1, Eli Ehrenpreis, MD, FACG2
1Advocate Lutheran General, Park Ridge, IL; 2Advocate Lutheran General, Evanston, IL
Introduction: Tofacitinib, an intracellular tyrosine kinase (Jak-Kinase) inhibitor, is approved by the U.S. Food and Drug Administration (FDA) for active rheumatoid arthritis (RA), psoriatic arthritis (PsA) and ulcerative colitis (UC). In 2019, the FDA released a safety alert for the risk of blood clots and death in patients receiving tofacitinib. In 2021, Black Box Warnings were issued for low dose (5mg BID) and high dose (10mg BID) tofacitinib, for increased risk of serious heart-related adverse events (AEs) and cancer.
Methods: FAERS is a database of voluntarily reported AEs used for post-marketing surveillance of medications. 8,863,077 FAERS reports from January 2018 to September 2021 were examined. Of these, there were 84,225 reports of tofacitinib-related AEs. Using MeDRA terminology, reports of cardiovascular (CV) AEs emphasized in recent black box warnings (Pulmonary Embolism, Deep Vein Thrombosis, Cerebral Thrombosis, Cerebral Venous Thrombosis, and Cerebral Vascular Occlusion) were reviewed. There were 650 CV AEs reported in the study period. Demographics, cumulative dosage, indications for drug use, outcomes, reactions, and reporter trends were analyzed. Reporter odds ratio (ROR) for all reporting groups (physicians, lawyers, community, pharmacists, etc.) were calculated. ROR >1 indicates interference in reporting.
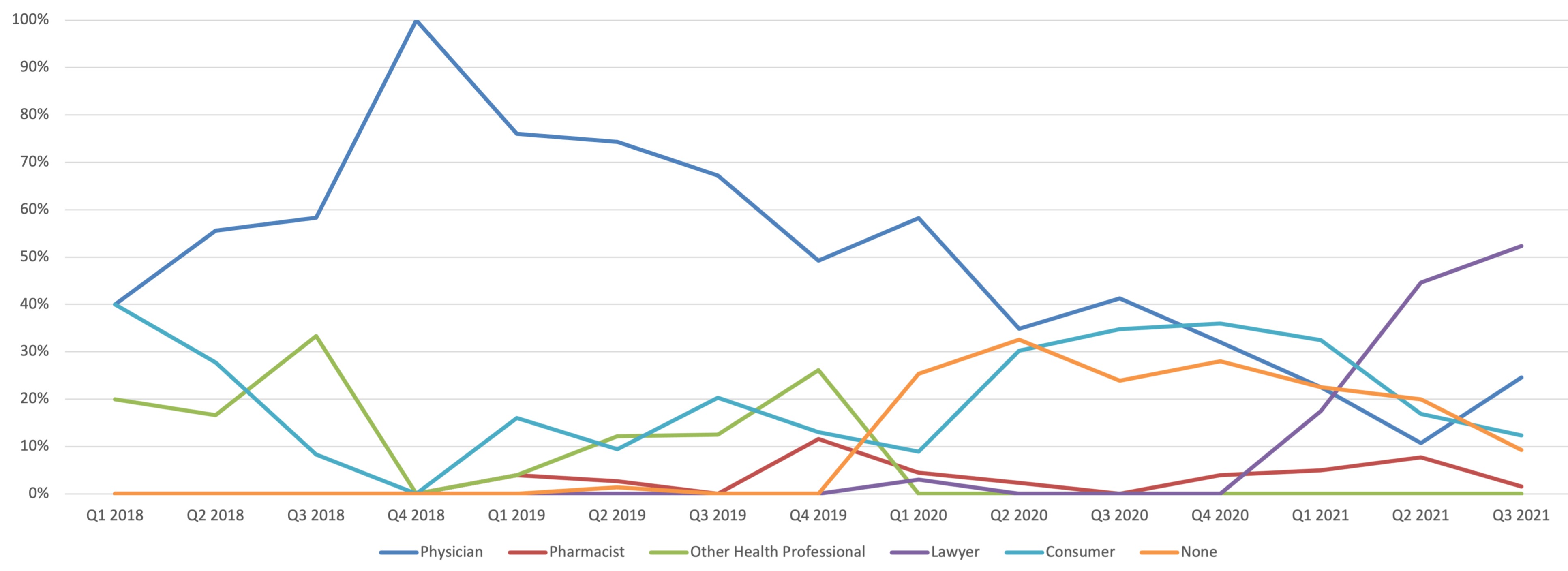
Results: The most common indication for tofacitinib was RA 43,386 (52%) of reports. Hospitalization occurred in 10,182/84,225 (12.0%) of reports. There were 3,856 (5%) reports when tofacitinib was indicated for UC. 42,671 (51%) of AEs occurred in subjects 36-64 years old. Females represented 65,806 (78%) of reports. Physicians were 13% of reporters while consumers accounted for 59% of reporters, (ROR = 1.52; 95%; CI = 1.49-1.54). CV specific AEs concerning Tofacitinib accounted for 650/84,225 (0.77%) of reports. Lawyer reporting demonstrated a sharp rise in 2021. Lawyer reports accounted for 70/170 (41%) CV AEs in 2021(ROR = 942; 95%; CI = 505-1757).
Discussion: Interference of the reporting of tofacitinib-related CV AEs to FAERS appears to be occurring, initially by excess consumer reporting and more recently by lawyers. The effect of reporting bias on the use of tofacitinib in the clinical setting requires further investigation.
Disclosures:
Sam D. Papasotiriou, BS1, Ryan Meader, DO1, Eli Ehrenpreis, MD, FACG2. C0384 - Tofacitinib-Associated Adverse Vascular Events Reported to the Federal Adverse Event Reporting System (FAERS) Database, ACG 2022 Annual Scientific Meeting Abstracts. Charlotte, NC: American College of Gastroenterology.
1Advocate Lutheran General, Park Ridge, IL; 2Advocate Lutheran General, Evanston, IL
Introduction: Tofacitinib, an intracellular tyrosine kinase (Jak-Kinase) inhibitor, is approved by the U.S. Food and Drug Administration (FDA) for active rheumatoid arthritis (RA), psoriatic arthritis (PsA) and ulcerative colitis (UC). In 2019, the FDA released a safety alert for the risk of blood clots and death in patients receiving tofacitinib. In 2021, Black Box Warnings were issued for low dose (5mg BID) and high dose (10mg BID) tofacitinib, for increased risk of serious heart-related adverse events (AEs) and cancer.
Methods: FAERS is a database of voluntarily reported AEs used for post-marketing surveillance of medications. 8,863,077 FAERS reports from January 2018 to September 2021 were examined. Of these, there were 84,225 reports of tofacitinib-related AEs. Using MeDRA terminology, reports of cardiovascular (CV) AEs emphasized in recent black box warnings (Pulmonary Embolism, Deep Vein Thrombosis, Cerebral Thrombosis, Cerebral Venous Thrombosis, and Cerebral Vascular Occlusion) were reviewed. There were 650 CV AEs reported in the study period. Demographics, cumulative dosage, indications for drug use, outcomes, reactions, and reporter trends were analyzed. Reporter odds ratio (ROR) for all reporting groups (physicians, lawyers, community, pharmacists, etc.) were calculated. ROR >1 indicates interference in reporting.
Results: The most common indication for tofacitinib was RA 43,386 (52%) of reports. Hospitalization occurred in 10,182/84,225 (12.0%) of reports. There were 3,856 (5%) reports when tofacitinib was indicated for UC. 42,671 (51%) of AEs occurred in subjects 36-64 years old. Females represented 65,806 (78%) of reports. Physicians were 13% of reporters while consumers accounted for 59% of reporters, (ROR = 1.52; 95%; CI = 1.49-1.54). CV specific AEs concerning Tofacitinib accounted for 650/84,225 (0.77%) of reports. Lawyer reporting demonstrated a sharp rise in 2021. Lawyer reports accounted for 70/170 (41%) CV AEs in 2021(ROR = 942; 95%; CI = 505-1757).
Discussion: Interference of the reporting of tofacitinib-related CV AEs to FAERS appears to be occurring, initially by excess consumer reporting and more recently by lawyers. The effect of reporting bias on the use of tofacitinib in the clinical setting requires further investigation.

Figure: Tofacitinib Cardiovascular AEs by Reporter Type
Disclosures:
Sam Papasotiriou indicated no relevant financial relationships.
Ryan Meader indicated no relevant financial relationships.
Eli Ehrenpreis: E2Bio Life Sciences – Owner/Ownership Interest, Stock-privately held company. Evanston Hospital – Consultant. Level-Ex – Consultant.
Sam D. Papasotiriou, BS1, Ryan Meader, DO1, Eli Ehrenpreis, MD, FACG2. C0384 - Tofacitinib-Associated Adverse Vascular Events Reported to the Federal Adverse Event Reporting System (FAERS) Database, ACG 2022 Annual Scientific Meeting Abstracts. Charlotte, NC: American College of Gastroenterology.
