Back


Poster Session A - Sunday Afternoon
Category: Colon
A0139 - The Use of Rituximab for IgG4–Related Sclerosing Mesenteritis
Sunday, October 23, 2022
5:00 PM – 7:00 PM ET
Location: Crown Ballroom

Has Audio

June Tome, MD
Mayo Clinic
Rochester, MN
Presenting Author(s)
June Tome, MD, Amrit K. Kamboj, MD, Darrell S. Pardi, MD, MS, FACG
Mayo Clinic, Rochester, MN
Introduction: Sclerosing mesenteritis (SM) is an uncommon fibro-inflammatory disease affecting the abdominal mesentery. Although some patients are asymptomatic or have minimal symptoms, SM can present with complications such as bowel obstruction, chylous ascites, and mesenteric ischemia. First-line therapy includes glucocorticoids in combination with tamoxifen in those who are symptomatic.
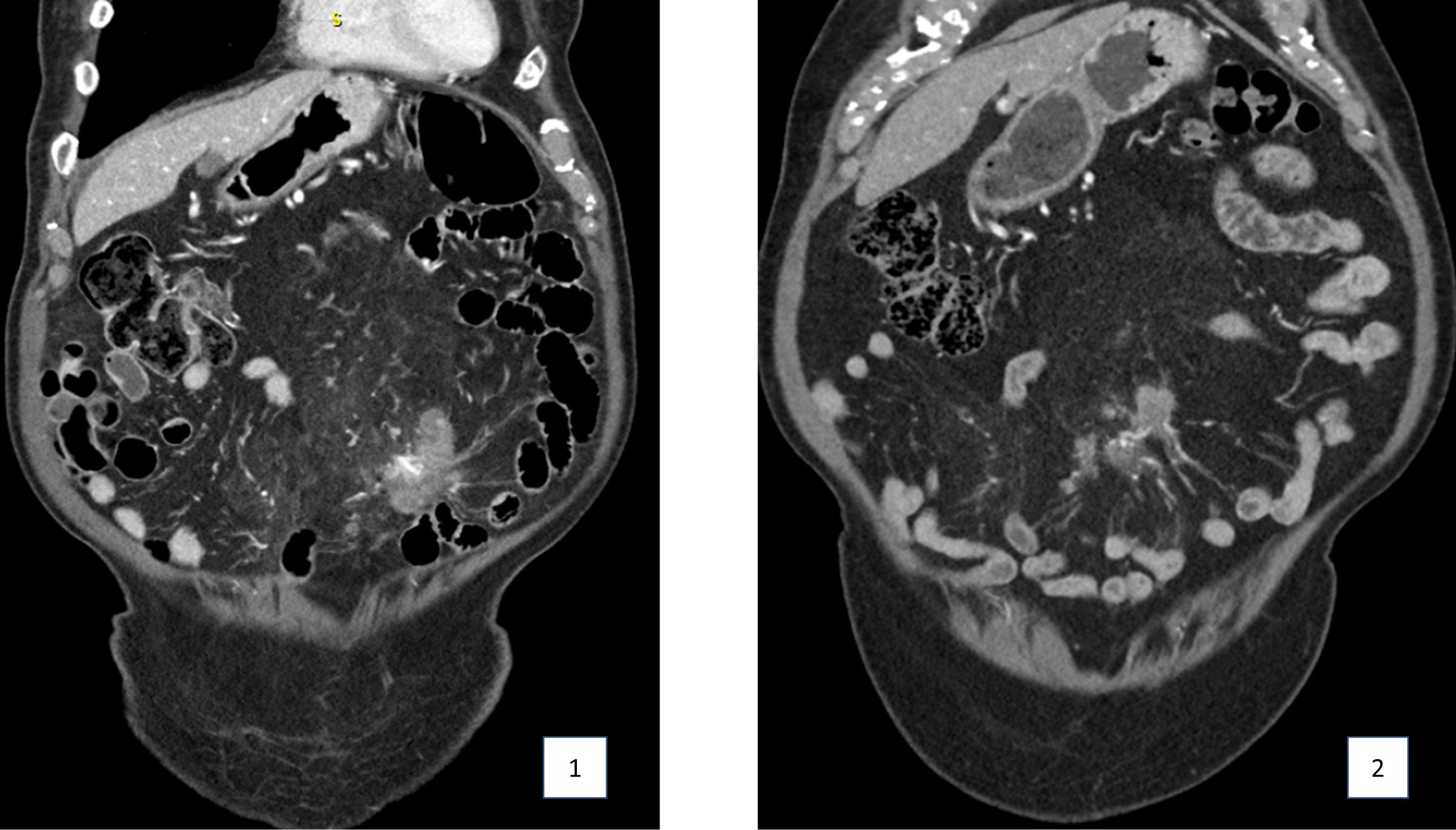
Case Description/Methods: An 82-year-old man with a history of prostate cancer and melanoma in remission and lymphocytic colitis presented with 4-months of poor appetite and 40-pound unintentional weight loss. Computed tomography (CT) abdomen showed an irregular 8.6 cm mesenteric mass with surrounding misty mesentery (Figure 1). CT-guided biopsy demonstrated fibro-adipose tissue with increased IgG4-positive plasma cells, supporting a diagnosis of IgG4-related SM. Follow-up CT abdomen 6 months later demonstrated enlargement of the mass with new encasement of the jejunal and ileal branches of the superior mesenteric artery and vein. Given impending mesenteric ischemia, he was treated with rituximab, a monoclonal anti-CD20 antibody, with two infusions two weeks apart without side-effects. He had contraindications to first-line therapy with glucocorticoids given prior suicidal ideation while on budesonide for microscopic colitis. Three months following treatment, his erythrocyte sedimentation rate improved from 52 to 25 (reference range, 3-28 mm/h) and IgG4 level from 851 to 267 (2.4-121 mg/dL). CT abdomen demonstrated a 50% decrease in the volume of the mesenteric mass without significant vascular involvement (Figure 2) and he had regained 30 pounds.
Discussion: Although rituximab has been studied for IgG4-related disease in general, the use of rituximab specifically for IgG4-related SM is not well-known. We report a case of a patient with IgG4-related SM treated effectively with rituximab, suggesting this may be a suitable medication for those who have contraindications or do not respond to current first-line therapy, especially if IgG4-related. Whether this drug would also work in patients with SM not related to IgG4 disease is unknown. Patients treated with rituximab should be closely monitored for infections, as well as allergic and infusion-related reactions.
Disclosures:
June Tome, MD, Amrit K. Kamboj, MD, Darrell S. Pardi, MD, MS, FACG. A0139 - The Use of Rituximab for IgG4–Related Sclerosing Mesenteritis, ACG 2022 Annual Scientific Meeting Abstracts. Charlotte, NC: American College of Gastroenterology.
Mayo Clinic, Rochester, MN
Introduction: Sclerosing mesenteritis (SM) is an uncommon fibro-inflammatory disease affecting the abdominal mesentery. Although some patients are asymptomatic or have minimal symptoms, SM can present with complications such as bowel obstruction, chylous ascites, and mesenteric ischemia. First-line therapy includes glucocorticoids in combination with tamoxifen in those who are symptomatic.
Case Description/Methods: An 82-year-old man with a history of prostate cancer and melanoma in remission and lymphocytic colitis presented with 4-months of poor appetite and 40-pound unintentional weight loss. Computed tomography (CT) abdomen showed an irregular 8.6 cm mesenteric mass with surrounding misty mesentery (Figure 1). CT-guided biopsy demonstrated fibro-adipose tissue with increased IgG4-positive plasma cells, supporting a diagnosis of IgG4-related SM. Follow-up CT abdomen 6 months later demonstrated enlargement of the mass with new encasement of the jejunal and ileal branches of the superior mesenteric artery and vein. Given impending mesenteric ischemia, he was treated with rituximab, a monoclonal anti-CD20 antibody, with two infusions two weeks apart without side-effects. He had contraindications to first-line therapy with glucocorticoids given prior suicidal ideation while on budesonide for microscopic colitis. Three months following treatment, his erythrocyte sedimentation rate improved from 52 to 25 (reference range, 3-28 mm/h) and IgG4 level from 851 to 267 (2.4-121 mg/dL). CT abdomen demonstrated a 50% decrease in the volume of the mesenteric mass without significant vascular involvement (Figure 2) and he had regained 30 pounds.
Discussion: Although rituximab has been studied for IgG4-related disease in general, the use of rituximab specifically for IgG4-related SM is not well-known. We report a case of a patient with IgG4-related SM treated effectively with rituximab, suggesting this may be a suitable medication for those who have contraindications or do not respond to current first-line therapy, especially if IgG4-related. Whether this drug would also work in patients with SM not related to IgG4 disease is unknown. Patients treated with rituximab should be closely monitored for infections, as well as allergic and infusion-related reactions.

Figure: CT abdomen pelvis of sclerosing mesenteritis before (figure 1) and after (figure 2) rituximab treatment.
Disclosures:
June Tome indicated no relevant financial relationships.
Amrit Kamboj indicated no relevant financial relationships.
Darrell Pardi: Abbvie – Consultant. Boehringer Ingelheim – Consultant. Ferring – Consultant. Finch – Grant/Research Support. Immunic – Consultant. Merck – Consultant. Otsuka – Consultant. Rebiotix – Grant/Research Support. Seres – Grant/Research Support. Takeda – Grant/Research Support. Vedanta – Consultant.
June Tome, MD, Amrit K. Kamboj, MD, Darrell S. Pardi, MD, MS, FACG. A0139 - The Use of Rituximab for IgG4–Related Sclerosing Mesenteritis, ACG 2022 Annual Scientific Meeting Abstracts. Charlotte, NC: American College of Gastroenterology.
