Back

Poster Session B - Monday Morning
Category: Colorectal Cancer Prevention
B0183 - A Rare Case of Duodenal Adenocarcinoma Discovered by Cologuard
Monday, October 24, 2022
10:00 AM – 12:00 PM ET
Location: Crown Ballroom

Has Audio

Shane Quo, DO
University of Miami Holy Cross Hospital
Fort Lauderdale, FL
Presenting Author(s)
Shane Quo, DO1, Mary Parianos, MD1, Howard Koch, MD2
1University of Miami Holy Cross Hospital, Fort Lauderdale, FL; 2Holy Cross Hospital, Fort Lauderdale, FL
Introduction: Non-invasive colon cancer screening is continuing to become more and more prominent in preventative medicine. According to the American Cancer Society, colorectal cancer is the third leading cause of cancer-related deaths in men and in women, although the death rate continues to decrease due to the increased rate of colon cancer screening. Cologuard has become another tool for decreasing the death rate of colon cancer. However, this statement proves true in a rare case of duodenal adenocarcinoma in a 74 year old female, which was diagnosed due to a positive Cologuard test.
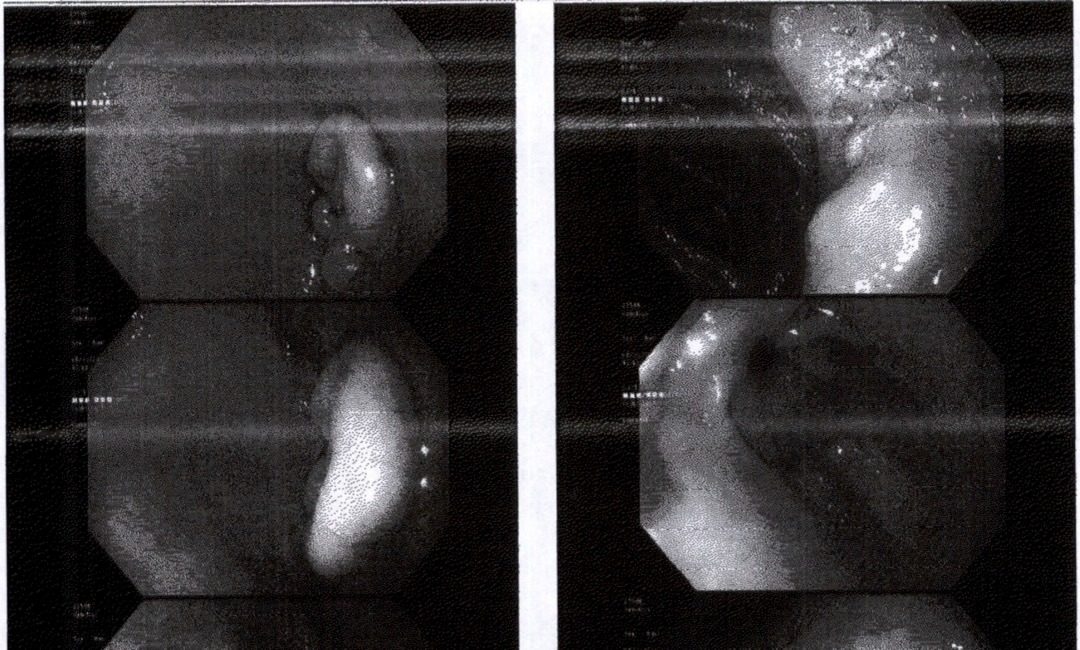
Case Description/Methods: A 74 year old woman with past medical history of invasive ductal carcinoma of the right breast and smoking history presents for an annual visitation. Due to her age and previous benign colon cancer screenings, her primary care physician opted for Cologuard for colorectal cancer screening, which returned positive. At this time, the patient complained of mild reflux symptoms, otherwise denied any other symptoms. Due to these symptoms and positive Cologuard, the patient was sent to see a gastroenterologist for evaluation. A colonoscopy and endoscopy due to the patient’s symptoms and Cologuard results. Colonoscopy was unremarkable. Endoscopy demonstrated a near circumferential adenomatous lesion in the first portion of the duodenum. Due to the size and extent of the lesion, endoscopic resection was not an option. A biopsy was performed and the pathology evaluation revealed that the duodenal mass was 1.8 cm well differentiated duodenal adenocarcinoma with invasion into the submucosa. The results prompted further imaging and referral to a general surgery for evaluation. General surgery performed a surgical resection of the duodenum with pancreaticoduodenectomy.
Discussion: There are minimal publications regarding small bowel carcinoma diagnosed due to a positive Cologuard. Our case report hopes to raise awareness of this possibility and inspire further research on the topic. Cologuard is an approved form of colon cancer screening that tests for blood and atypical DNA within stool. Following any abnormal results with this initial screening, it is recommended that the patient undergo a colonoscopy. However, at this time, there is limited published data referencing any benefit with Cologuard towards the finding of duodenal adenocarcinoma. This fact is precisely why this case is unique and raises the possibility for future study of Cologuard’s ability to be expanded to detection of carcinoma of the small bowel.
Disclosures:
Shane Quo, DO1, Mary Parianos, MD1, Howard Koch, MD2. B0183 - A Rare Case of Duodenal Adenocarcinoma Discovered by Cologuard, ACG 2022 Annual Scientific Meeting Abstracts. Charlotte, NC: American College of Gastroenterology.
1University of Miami Holy Cross Hospital, Fort Lauderdale, FL; 2Holy Cross Hospital, Fort Lauderdale, FL
Introduction: Non-invasive colon cancer screening is continuing to become more and more prominent in preventative medicine. According to the American Cancer Society, colorectal cancer is the third leading cause of cancer-related deaths in men and in women, although the death rate continues to decrease due to the increased rate of colon cancer screening. Cologuard has become another tool for decreasing the death rate of colon cancer. However, this statement proves true in a rare case of duodenal adenocarcinoma in a 74 year old female, which was diagnosed due to a positive Cologuard test.
Case Description/Methods: A 74 year old woman with past medical history of invasive ductal carcinoma of the right breast and smoking history presents for an annual visitation. Due to her age and previous benign colon cancer screenings, her primary care physician opted for Cologuard for colorectal cancer screening, which returned positive. At this time, the patient complained of mild reflux symptoms, otherwise denied any other symptoms. Due to these symptoms and positive Cologuard, the patient was sent to see a gastroenterologist for evaluation. A colonoscopy and endoscopy due to the patient’s symptoms and Cologuard results. Colonoscopy was unremarkable. Endoscopy demonstrated a near circumferential adenomatous lesion in the first portion of the duodenum. Due to the size and extent of the lesion, endoscopic resection was not an option. A biopsy was performed and the pathology evaluation revealed that the duodenal mass was 1.8 cm well differentiated duodenal adenocarcinoma with invasion into the submucosa. The results prompted further imaging and referral to a general surgery for evaluation. General surgery performed a surgical resection of the duodenum with pancreaticoduodenectomy.
Discussion: There are minimal publications regarding small bowel carcinoma diagnosed due to a positive Cologuard. Our case report hopes to raise awareness of this possibility and inspire further research on the topic. Cologuard is an approved form of colon cancer screening that tests for blood and atypical DNA within stool. Following any abnormal results with this initial screening, it is recommended that the patient undergo a colonoscopy. However, at this time, there is limited published data referencing any benefit with Cologuard towards the finding of duodenal adenocarcinoma. This fact is precisely why this case is unique and raises the possibility for future study of Cologuard’s ability to be expanded to detection of carcinoma of the small bowel.

Figure: Figure 1. Duodenal Adenocarcinoma on Endoscopy
Disclosures:
Shane Quo indicated no relevant financial relationships.
Mary Parianos indicated no relevant financial relationships.
Howard Koch indicated no relevant financial relationships.
Shane Quo, DO1, Mary Parianos, MD1, Howard Koch, MD2. B0183 - A Rare Case of Duodenal Adenocarcinoma Discovered by Cologuard, ACG 2022 Annual Scientific Meeting Abstracts. Charlotte, NC: American College of Gastroenterology.
