Back


Poster Session C - Monday Afternoon
Category: IBD
C0420 - Impact of Biologic Therapies on Risk of Major Adverse Cardiovascular Events in Crohn’s Disease: Systematic Review and Meta-Analysis of Randomized Controlled Trials
Monday, October 24, 2022
3:00 PM – 5:00 PM ET
Location: Crown Ballroom

Has Audio

Mohammad Shehab, MD
Kuwait University
Al-jabreyah, Hawalli, Kuwait
Presenting Author(s)
Mohammad Shehab, MD1, Afrah Alkazemi, PharmD2, Fatema Alrashed, PharmD1, Peter Lakatos, MD3, Talat Bessissow, MD3
1Kuwait University, Al-jabreyah, Hawalli, Kuwait; 2Kuwait university, Al-jabreyah, Hawalli, Kuwait; 3McGill University, Montreal, PQ, Canada
Introduction: Biologic therapies are efficacious in inducing response and remission in patients with moderate to severe Crohn’s Disease (CD); however, no previous systematic reviews investigated risk of major adverse cardiovascular events (MACEs). The aim of this systematic review and meta-analysis is to estimate the risk of MACEs in adult patients with CD treated with biologic therapies.
Methods: We systematically searched Medline, Cochrane Central Register of Controlled Trials, Scopus, and Embase databases up to March 2022 to identify eligible studies that assessed the risk of MACEs in adult patients (age ≥18 years) with CD on biologic therapies. Only phase 3, active-comparator or placebo controlled randomized trials (RCTs) were included in the analysis. Our primary outcome was the rate of MACEs observed in patients receiving biologic therapies during induction and maintenance phases of RCTs. Random effects model was used to calculate pooled odds ratios (ORs) and 95% CIs and I2 statistics was used to assess heterogeneity.
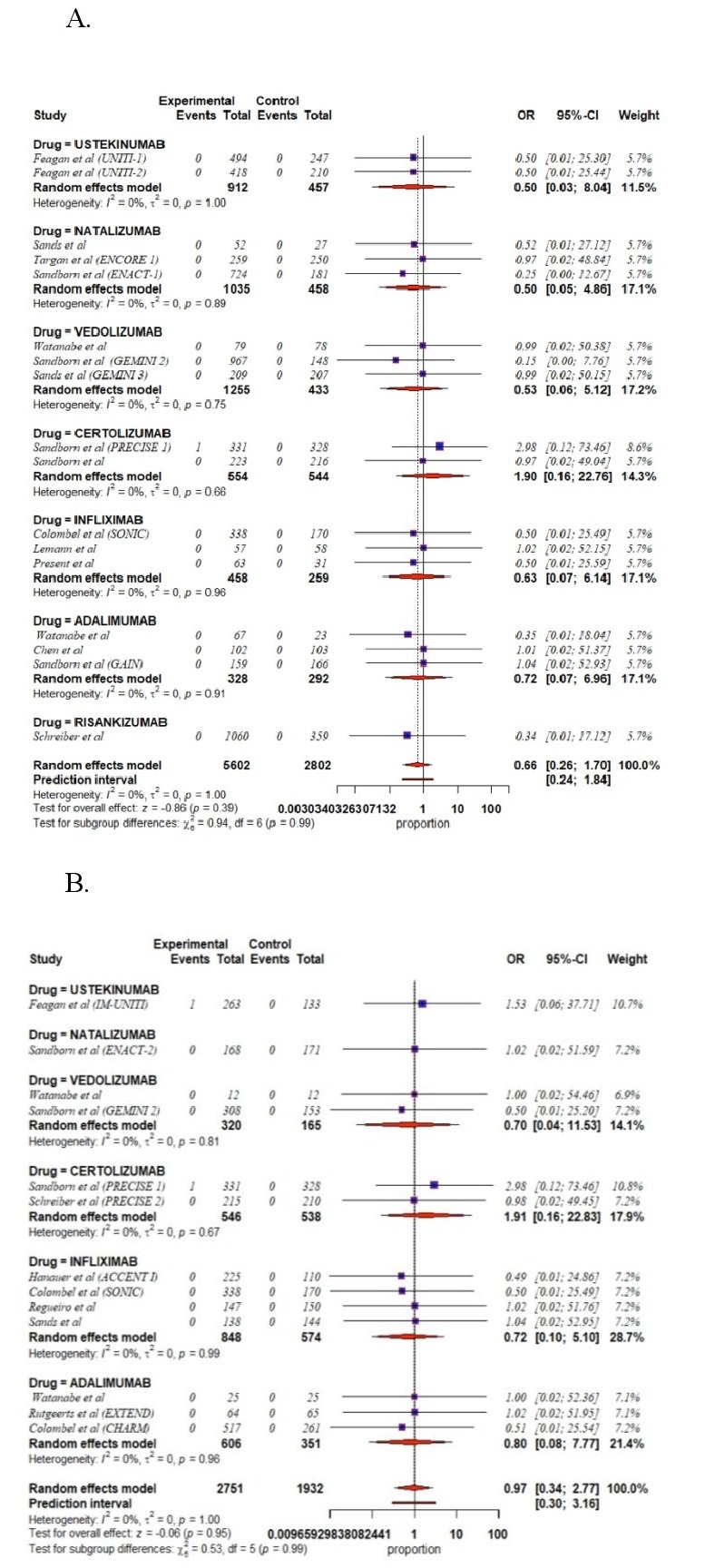
Results: Twenty-nine RCTs involving 12196 patients with CD were included in our systematic review and meta-analysis. There was no evidence of statistical heterogeneity across the studies using the I2 statistic (I2=0). Biologic therapies were not associated with increased risk of MACEs during induction [infliximab (OR 0.63, 95% CI 0.07–6.14), adalimumab (OR 0.72, 95% CI 0.07–6.96), ustekinumab (OR 0.50, 95% CI 0.03–8.04), natalizumab (OR 0.50, 95% CI 0.05–4.86), vedolizumab (OR 0.53, 95% CI 0.06–5.12), certolizumab (OR 1.90, 95% CI 0.16–22.76) and risankizumab (OR 0.34, 95% CI 0.01–17.12)]. Additionally, there was no statistically significant difference in risk of MACEs associated with the use of biologic therapies during maintenance compared to placebo [infliximab (OR 0.72, 95% CI 0.01–5.10), adalimumab (OR 0.80, 95% CI 0.08–7.77), ustekinumab (OR 1.53, 95% CI 0.06–37.71), natalizumab (OR 1.02, 95% CI 0.02–51.59), vedolizumab (OR 0.70 95% CI 0.04–11.53), and certolizumab (OR 1.91, 95% CI 0.16–22.83].
Discussion: Although pervious systematic reviews have studied the efficacy of biologic therapies in CD, major adverse cardiovascular events (MACEs), which are important safety outcomes, have not been addressed. In our study, we found that the use of biologic therapies among adult patients with Crohn’s disease was not associated with increased risk of MACEs. However, patient level data were lacking and meta-regression analyses were not performed to adjust for confounding factors.
Disclosures:
Mohammad Shehab, MD1, Afrah Alkazemi, PharmD2, Fatema Alrashed, PharmD1, Peter Lakatos, MD3, Talat Bessissow, MD3. C0420 - Impact of Biologic Therapies on Risk of Major Adverse Cardiovascular Events in Crohn’s Disease: Systematic Review and Meta-Analysis of Randomized Controlled Trials, ACG 2022 Annual Scientific Meeting Abstracts. Charlotte, NC: American College of Gastroenterology.
1Kuwait University, Al-jabreyah, Hawalli, Kuwait; 2Kuwait university, Al-jabreyah, Hawalli, Kuwait; 3McGill University, Montreal, PQ, Canada
Introduction: Biologic therapies are efficacious in inducing response and remission in patients with moderate to severe Crohn’s Disease (CD); however, no previous systematic reviews investigated risk of major adverse cardiovascular events (MACEs). The aim of this systematic review and meta-analysis is to estimate the risk of MACEs in adult patients with CD treated with biologic therapies.
Methods: We systematically searched Medline, Cochrane Central Register of Controlled Trials, Scopus, and Embase databases up to March 2022 to identify eligible studies that assessed the risk of MACEs in adult patients (age ≥18 years) with CD on biologic therapies. Only phase 3, active-comparator or placebo controlled randomized trials (RCTs) were included in the analysis. Our primary outcome was the rate of MACEs observed in patients receiving biologic therapies during induction and maintenance phases of RCTs. Random effects model was used to calculate pooled odds ratios (ORs) and 95% CIs and I2 statistics was used to assess heterogeneity.
Results: Twenty-nine RCTs involving 12196 patients with CD were included in our systematic review and meta-analysis. There was no evidence of statistical heterogeneity across the studies using the I2 statistic (I2=0). Biologic therapies were not associated with increased risk of MACEs during induction [infliximab (OR 0.63, 95% CI 0.07–6.14), adalimumab (OR 0.72, 95% CI 0.07–6.96), ustekinumab (OR 0.50, 95% CI 0.03–8.04), natalizumab (OR 0.50, 95% CI 0.05–4.86), vedolizumab (OR 0.53, 95% CI 0.06–5.12), certolizumab (OR 1.90, 95% CI 0.16–22.76) and risankizumab (OR 0.34, 95% CI 0.01–17.12)]. Additionally, there was no statistically significant difference in risk of MACEs associated with the use of biologic therapies during maintenance compared to placebo [infliximab (OR 0.72, 95% CI 0.01–5.10), adalimumab (OR 0.80, 95% CI 0.08–7.77), ustekinumab (OR 1.53, 95% CI 0.06–37.71), natalizumab (OR 1.02, 95% CI 0.02–51.59), vedolizumab (OR 0.70 95% CI 0.04–11.53), and certolizumab (OR 1.91, 95% CI 0.16–22.83].
Discussion: Although pervious systematic reviews have studied the efficacy of biologic therapies in CD, major adverse cardiovascular events (MACEs), which are important safety outcomes, have not been addressed. In our study, we found that the use of biologic therapies among adult patients with Crohn’s disease was not associated with increased risk of MACEs. However, patient level data were lacking and meta-regression analyses were not performed to adjust for confounding factors.

Figure: A. Forest plot showing risk of MACEs in patients receiving biologic therapies for the induction of remission in randomized controlled trials
B. Forest plot showing risk of MACEs in patients receiving biologic therapies for the maintenance of remission in randomized controlled trials
B. Forest plot showing risk of MACEs in patients receiving biologic therapies for the maintenance of remission in randomized controlled trials
Disclosures:
Mohammad Shehab indicated no relevant financial relationships.
Afrah Alkazemi indicated no relevant financial relationships.
Fatema Alrashed indicated no relevant financial relationships.
Peter Lakatos indicated no relevant financial relationships.
Talat Bessissow indicated no relevant financial relationships.
Mohammad Shehab, MD1, Afrah Alkazemi, PharmD2, Fatema Alrashed, PharmD1, Peter Lakatos, MD3, Talat Bessissow, MD3. C0420 - Impact of Biologic Therapies on Risk of Major Adverse Cardiovascular Events in Crohn’s Disease: Systematic Review and Meta-Analysis of Randomized Controlled Trials, ACG 2022 Annual Scientific Meeting Abstracts. Charlotte, NC: American College of Gastroenterology.
