Back


Poster Session C - Monday Afternoon
Category: Liver
C0497 - Therapeutic Effect of Granulocyte Colony Stimulating Factor Therapy on Patients With Cirrhosis: A Systematic Review and Meta-Analysis
Monday, October 24, 2022
3:00 PM – 5:00 PM ET
Location: Crown Ballroom

Has Audio
- JZ
Javaid Zafar
Lahore General Hospital
Lahore, Punjab, Pakistan
Presenting Author(s)
Javaid Zafar, 1, Syed Sarmad Javaid, 2, Ahmed Kamal Siddiqi, 3, Adnan Zafar, MBBS4, Ahmed Mustafa Rashid, 2, Arsalan Zafar Iqbal, MBBS5, Muhammad Waqas Tahir, MD6, Yousaf Zafar, 7
1Lahore General Hospital, Lahore, Punjab, Pakistan; 2Jinnah Sindh Medical University, Karachi, Sindh, Pakistan; 3Ziauddin Medical University, Karachi, Sindh, Pakistan; 4CMH Lahore Medical College, Lahore, Punjab, Pakistan; 5FMH Lahore Medical College, Lahore, Punjab, Pakistan; 6Rochester General Hospital, Rochester, NY; 7University of Mississippi Medical Center, Madison, MS
Introduction: Decompensated cirrhosis is an advanced stage of cirrhosis in which liver scarring becomes so extensive that the liver is unable to function properly, leading to complications such as refractory ascites, recurrent infections, and hepatic encephalopathy. Currently, liver transplantation is the only definitive treatment, but it has a number of disadvantages, including high cost, restricted donor pool, and long-term immunosuppression. As a result, granulocyte colony stimulating factor (G-CSF) has emerged as an alternative therapy. However, its clinical efficacy is still debatable, so the aim of this meta-analysis was to determine the efficacy of G-CSF in patients with cirrhosis.
Methods: MEDLINE and SCOPUS were queried from inception till June 2022 for randomized controlled trials (RCTs) without any restrictions. RCTs examining the impact of G-CSF on survival rates in patients with decompensated cirrhosis and compensated cirrhosis were incorporated. The results were reported using a random-effects meta-analysis and the Mantel-Haenszel risk ratio (RR). A P-value of < 0.05 was considered significant for the analysis. The subgroup analysis was performed to investigate the influence of study-level variables such as etiology on outcomes of interest.
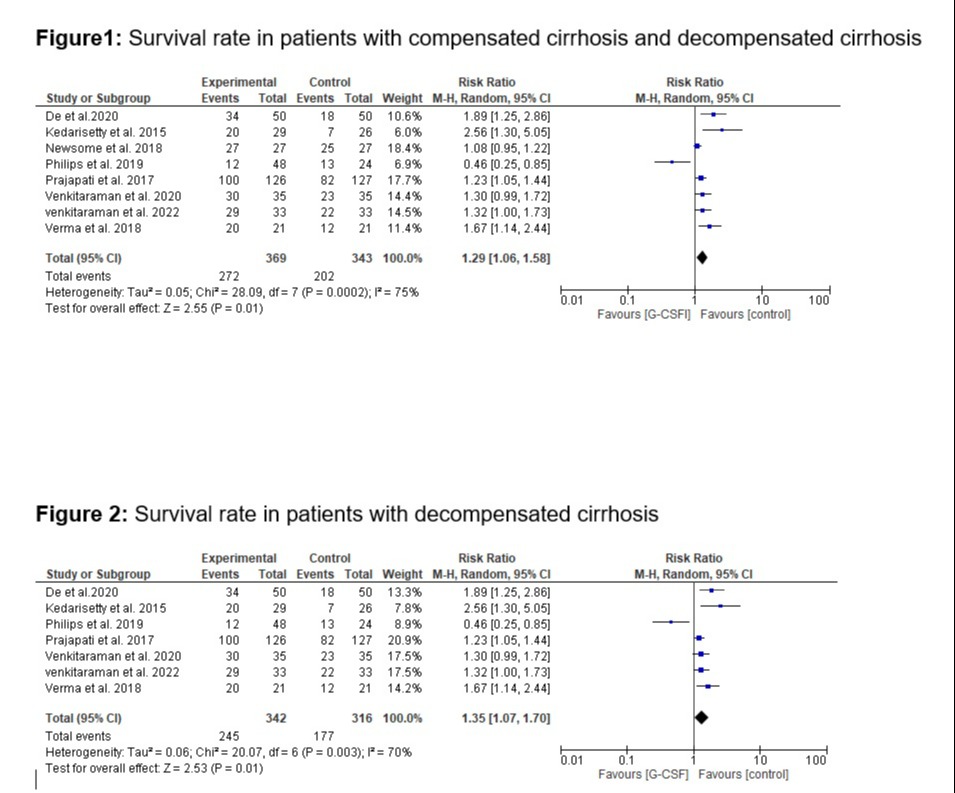
Results: Eight studies (n = 8) were included in our meta-analysis. The total number of participants in our study was 712, and the median study duration was 12 months. Our pooled analysis demonstrates that G-CSF treatment significantly improved survival rates (RR 1.29; 95% CI 1.06 to 1.58; p = 0.01; Figure 1) in patients with compensated cirrhosis and decompensated cirrhosis. In our subgroup analysis, G-CSF was also linked to higher survival rates among people with decompensated cirrhosis (RR 1.35; 95% CI 1.07 to 1.70; p = 0.01; Figure 2).
Discussion: Our findings indicate that G-CSF treatment is successful in improving the survival rates in patients with decompensated cirrhosis and compensated cirrhosis. Hence, it can be employed as an alternative therapeutic option.
Disclosures:
Javaid Zafar, 1, Syed Sarmad Javaid, 2, Ahmed Kamal Siddiqi, 3, Adnan Zafar, MBBS4, Ahmed Mustafa Rashid, 2, Arsalan Zafar Iqbal, MBBS5, Muhammad Waqas Tahir, MD6, Yousaf Zafar, 7. C0497 - Therapeutic Effect of Granulocyte Colony Stimulating Factor Therapy on Patients With Cirrhosis: A Systematic Review and Meta-Analysis, ACG 2022 Annual Scientific Meeting Abstracts. Charlotte, NC: American College of Gastroenterology.
1Lahore General Hospital, Lahore, Punjab, Pakistan; 2Jinnah Sindh Medical University, Karachi, Sindh, Pakistan; 3Ziauddin Medical University, Karachi, Sindh, Pakistan; 4CMH Lahore Medical College, Lahore, Punjab, Pakistan; 5FMH Lahore Medical College, Lahore, Punjab, Pakistan; 6Rochester General Hospital, Rochester, NY; 7University of Mississippi Medical Center, Madison, MS
Introduction: Decompensated cirrhosis is an advanced stage of cirrhosis in which liver scarring becomes so extensive that the liver is unable to function properly, leading to complications such as refractory ascites, recurrent infections, and hepatic encephalopathy. Currently, liver transplantation is the only definitive treatment, but it has a number of disadvantages, including high cost, restricted donor pool, and long-term immunosuppression. As a result, granulocyte colony stimulating factor (G-CSF) has emerged as an alternative therapy. However, its clinical efficacy is still debatable, so the aim of this meta-analysis was to determine the efficacy of G-CSF in patients with cirrhosis.
Methods: MEDLINE and SCOPUS were queried from inception till June 2022 for randomized controlled trials (RCTs) without any restrictions. RCTs examining the impact of G-CSF on survival rates in patients with decompensated cirrhosis and compensated cirrhosis were incorporated. The results were reported using a random-effects meta-analysis and the Mantel-Haenszel risk ratio (RR). A P-value of < 0.05 was considered significant for the analysis. The subgroup analysis was performed to investigate the influence of study-level variables such as etiology on outcomes of interest.
Results: Eight studies (n = 8) were included in our meta-analysis. The total number of participants in our study was 712, and the median study duration was 12 months. Our pooled analysis demonstrates that G-CSF treatment significantly improved survival rates (RR 1.29; 95% CI 1.06 to 1.58; p = 0.01; Figure 1) in patients with compensated cirrhosis and decompensated cirrhosis. In our subgroup analysis, G-CSF was also linked to higher survival rates among people with decompensated cirrhosis (RR 1.35; 95% CI 1.07 to 1.70; p = 0.01; Figure 2).
Discussion: Our findings indicate that G-CSF treatment is successful in improving the survival rates in patients with decompensated cirrhosis and compensated cirrhosis. Hence, it can be employed as an alternative therapeutic option.

Figure: Forrest Plots of Analysis
Disclosures:
Javaid Zafar indicated no relevant financial relationships.
Syed Sarmad Javaid indicated no relevant financial relationships.
Ahmed Kamal Siddiqi indicated no relevant financial relationships.
Adnan Zafar indicated no relevant financial relationships.
Ahmed Mustafa Rashid indicated no relevant financial relationships.
Arsalan Zafar Iqbal indicated no relevant financial relationships.
Muhammad Waqas Tahir indicated no relevant financial relationships.
Yousaf Zafar indicated no relevant financial relationships.
Javaid Zafar, 1, Syed Sarmad Javaid, 2, Ahmed Kamal Siddiqi, 3, Adnan Zafar, MBBS4, Ahmed Mustafa Rashid, 2, Arsalan Zafar Iqbal, MBBS5, Muhammad Waqas Tahir, MD6, Yousaf Zafar, 7. C0497 - Therapeutic Effect of Granulocyte Colony Stimulating Factor Therapy on Patients With Cirrhosis: A Systematic Review and Meta-Analysis, ACG 2022 Annual Scientific Meeting Abstracts. Charlotte, NC: American College of Gastroenterology.
