Back


Poster Session A - Sunday Afternoon
Category: Liver
A0511 - A Potential New Use for Tocilizumab: A Case of Refractory Checkpoint Inhibitor Hepatitis
Sunday, October 23, 2022
5:00 PM – 7:00 PM ET
Location: Crown Ballroom

Has Audio

Laine A. Lyles, DO
Prisma Health-Midlands/ University of South Carolina
Lexington, SC
Presenting Author(s)
Laine A. Lyles, DO1, Reagan Farmer, 2
1Prisma Health-Midlands/ University of South Carolina, Lexington, SC; 2University of South Carolina School of Medicine, Columbia, SC
Introduction: Immune checkpoint inhibitors (CPI) are becoming increasingly common treatment options for several types of cancers. While their use in cancer treatment is very promising, these medications are not without side effects. Specifically, these drugs are commonly associated with hepatic adverse events, including auto-immune hepatitis (AIH). In fact, in one study, AIH was found in approximately 5% of people treated with a CPI. While we know that CPI hepatitis is a potential outcome of treatment with CPIs, we have yet to establish the best treatment options for this adverse event. Based on expert opinion, the recommended course of treatment involves high dose steroids and immunomodulators if needed. There is not, however, much literature on management of CPI hepatitis that is refractory to the typical treatments, though use of tocilizumab has shown some success previously in treatment of immune-related adverse events secondary to CPIs .
Case Description/Methods: A 35 y.o. female with recurrent right renal cell carcinoma, status-post resection with nephrectomy, on palliative treatment with nivolumab/ipilimumab, presented to the hospital for further evaluation of elevated liver function tests (LFT) and an abdominal MRI concerning for hepatitis. At time of admission, she was on her 3rd week of steroids, started with concern for drug-induced autoimmune hepatitis due to her checkpoint inhibitor (CPI) treatment. At admission, she was started on IV steroids and mycophenolic acid. LFTs and bilirubin continued to rise despite several days of this treatment and she was given a dose of rituximab. Liver biopsy was obtained which was consistent with CPI induced hepatitis. LFTs and bilirubin continued to rise and tocilizumab was given. 4 days after that treatment, AST and ALT were nearly half their pretreatment values and bilirubin began downtrending. At this point, the patient was stable enough for discharge with very close follow up.
Discussion: Studies from 2019 reveal that 43.68% of all people with cancer in the United States are eligible for CPI therapy. This number translates to 7.9 million patients who are potentially eligible for CPI therapy. With the incidence of severe CPI hepatitis suspected to be up to 20% of all people taking CPIs, this equates to 1.58 million people of whom could be affected. More studies need to be done to address treatment for refractory auto-immune CPI hepatitis, and to better understand the underlying pathogenesis in this rapidly growing population.
Disclosures:
Laine A. Lyles, DO1, Reagan Farmer, 2. A0511 - A Potential New Use for Tocilizumab: A Case of Refractory Checkpoint Inhibitor Hepatitis, ACG 2022 Annual Scientific Meeting Abstracts. Charlotte, NC: American College of Gastroenterology.
1Prisma Health-Midlands/ University of South Carolina, Lexington, SC; 2University of South Carolina School of Medicine, Columbia, SC
Introduction: Immune checkpoint inhibitors (CPI) are becoming increasingly common treatment options for several types of cancers. While their use in cancer treatment is very promising, these medications are not without side effects. Specifically, these drugs are commonly associated with hepatic adverse events, including auto-immune hepatitis (AIH). In fact, in one study, AIH was found in approximately 5% of people treated with a CPI. While we know that CPI hepatitis is a potential outcome of treatment with CPIs, we have yet to establish the best treatment options for this adverse event. Based on expert opinion, the recommended course of treatment involves high dose steroids and immunomodulators if needed. There is not, however, much literature on management of CPI hepatitis that is refractory to the typical treatments, though use of tocilizumab has shown some success previously in treatment of immune-related adverse events secondary to CPIs .
Case Description/Methods: A 35 y.o. female with recurrent right renal cell carcinoma, status-post resection with nephrectomy, on palliative treatment with nivolumab/ipilimumab, presented to the hospital for further evaluation of elevated liver function tests (LFT) and an abdominal MRI concerning for hepatitis. At time of admission, she was on her 3rd week of steroids, started with concern for drug-induced autoimmune hepatitis due to her checkpoint inhibitor (CPI) treatment. At admission, she was started on IV steroids and mycophenolic acid. LFTs and bilirubin continued to rise despite several days of this treatment and she was given a dose of rituximab. Liver biopsy was obtained which was consistent with CPI induced hepatitis. LFTs and bilirubin continued to rise and tocilizumab was given. 4 days after that treatment, AST and ALT were nearly half their pretreatment values and bilirubin began downtrending. At this point, the patient was stable enough for discharge with very close follow up.
Discussion: Studies from 2019 reveal that 43.68% of all people with cancer in the United States are eligible for CPI therapy. This number translates to 7.9 million patients who are potentially eligible for CPI therapy. With the incidence of severe CPI hepatitis suspected to be up to 20% of all people taking CPIs, this equates to 1.58 million people of whom could be affected. More studies need to be done to address treatment for refractory auto-immune CPI hepatitis, and to better understand the underlying pathogenesis in this rapidly growing population.

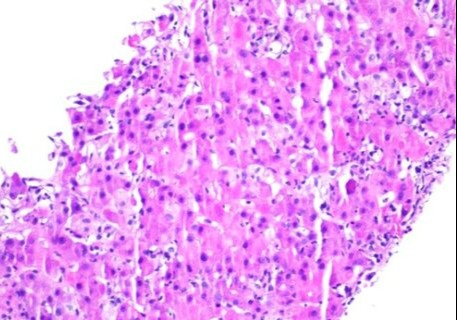
Figure: Liver biopsy with significant lobular hepatitis, loss of zone 3 hepatocytes, acidophil bodies, and Kupffer cell hyperplasia consistant with checkpoint inhibitor auto-immune hepatitis.
Disclosures:
Laine Lyles indicated no relevant financial relationships.
Reagan Farmer indicated no relevant financial relationships.
Laine A. Lyles, DO1, Reagan Farmer, 2. A0511 - A Potential New Use for Tocilizumab: A Case of Refractory Checkpoint Inhibitor Hepatitis, ACG 2022 Annual Scientific Meeting Abstracts. Charlotte, NC: American College of Gastroenterology.

