Back

Poster Session C - Monday Afternoon
Category: Liver
C0525 - Federal Mandates and Trends in Acetaminophen-Opioid Combination Product Supratherapeutic Ingestions at a Large Urban Safety-Net Hospital From 2011-2020
Monday, October 24, 2022
3:00 PM – 5:00 PM ET
Location: Crown Ballroom

Has Audio

Elizabeth Harris, MD
UT Southwestern Medical Center
Lexington, KY
Presenting Author(s)
Elizabeth Harris, MD1, Michael Harms, MS2, Dazhe Cao, MD3, Courtney Prestwood, MD4, Lister DeBinya, BS1, Kurt Kleinschmidt, MD1, Amy Young, MD5, Srishti Saha, MD1, Jody Rule, PhD1, Kristin Alvarez, PharmD2, Sandeep R. Das, MD, MPH1, William Lee, MD1
1UT Southwestern Medical Center, Dallas, TX; 2Parkland Hospital, Dallas, TX; 3University of Texas Southwestern Medical Center, Dallas, TX; 4University of Texas Southwestern Medical School, Galveston, TX; 5UT Southwestern, Dallas, TX
Introduction: Acetaminophen-opioid (APAP-opioid) combination products are ubiquitously used for pain relief. During the ongoing opioid epidemic, people have misused these products causing cases of hepatotoxicity. In 2014, the US Food and Drug Administration limited the amount of acetaminophen (APAP) in combination products to 325 mg, and the US Drug Enforcement Administration changed hydrocodone/APAP from schedule III to schedule II. This project aims to determine whether these federal mandates led to changes in APAP-opioid supratherapeutic ingestions at a large safety-net hospital from 2011-2020.
Methods: Hospital encounters between 1/1/2011-12/31/2020 of patients ≥ 18 years old with reported supratherapeutic ingestions and a detectable APAP level ( > 10 mcg/mL) were identified from our electronic health record. We manually screened charts for encounters involving APAP-opioid products and from these extracted demographic information, laboratory values, product(s) ingested, ingestion intent and amount, and disposition. We describe the number of encounters per year.
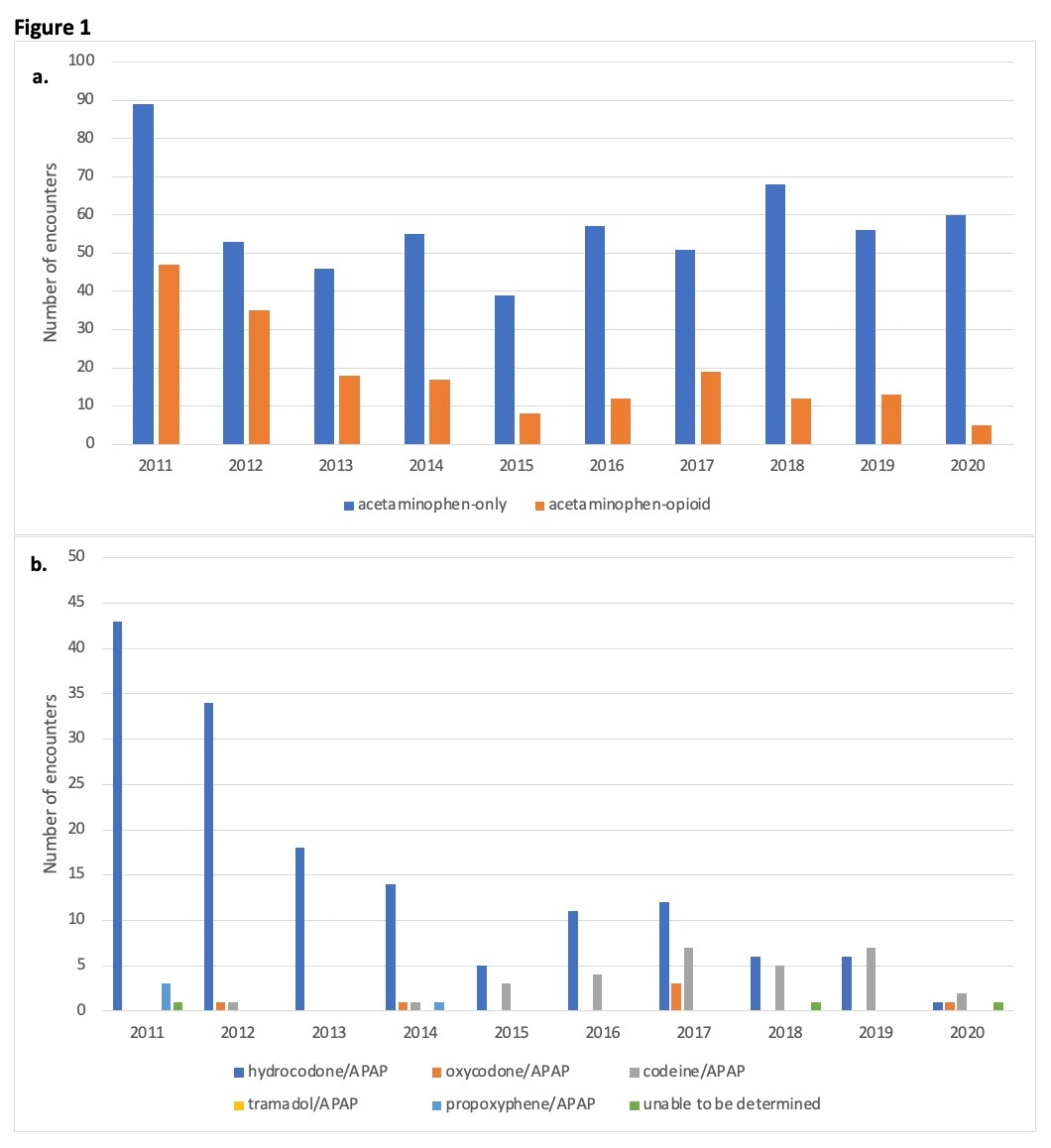
Results: Of 760 encounters, 186 (25%) involved APAP-opioid combination products. Most patients were Caucasian (83%), non-Hispanic (76%), and female (54%). The number of supratherapeutic APAP-opioid ingestions decreased over the 10-year period (Fig.1a). Most ingestions were acute (64%) and with intent at self-harm (57%). Most patients were discharged from the Emergency Department (37%) and not treated with N-acetylcysteine (65%). Only five cases developed acute liver failure. The most used combination product was hydrocodone/APAP (80%). A downtrend in hydrocodone/APAP accompanied a relative increase in codeine/APAP ingestions from 2015 onwards (Fig.1b).
Discussion: Our study shows that during the past 10 years the number of APAP-opioid supratherapeutic ingestions declined, especially hydrocodone/APAP likely at least in part due to its re-classification to schedule II. Codeine, schedule III, may have replaced some hydrocodone prescriptions.
Disclosures:
Elizabeth Harris, MD1, Michael Harms, MS2, Dazhe Cao, MD3, Courtney Prestwood, MD4, Lister DeBinya, BS1, Kurt Kleinschmidt, MD1, Amy Young, MD5, Srishti Saha, MD1, Jody Rule, PhD1, Kristin Alvarez, PharmD2, Sandeep R. Das, MD, MPH1, William Lee, MD1. C0525 - Federal Mandates and Trends in Acetaminophen-Opioid Combination Product Supratherapeutic Ingestions at a Large Urban Safety-Net Hospital From 2011-2020, ACG 2022 Annual Scientific Meeting Abstracts. Charlotte, NC: American College of Gastroenterology.
1UT Southwestern Medical Center, Dallas, TX; 2Parkland Hospital, Dallas, TX; 3University of Texas Southwestern Medical Center, Dallas, TX; 4University of Texas Southwestern Medical School, Galveston, TX; 5UT Southwestern, Dallas, TX
Introduction: Acetaminophen-opioid (APAP-opioid) combination products are ubiquitously used for pain relief. During the ongoing opioid epidemic, people have misused these products causing cases of hepatotoxicity. In 2014, the US Food and Drug Administration limited the amount of acetaminophen (APAP) in combination products to 325 mg, and the US Drug Enforcement Administration changed hydrocodone/APAP from schedule III to schedule II. This project aims to determine whether these federal mandates led to changes in APAP-opioid supratherapeutic ingestions at a large safety-net hospital from 2011-2020.
Methods: Hospital encounters between 1/1/2011-12/31/2020 of patients ≥ 18 years old with reported supratherapeutic ingestions and a detectable APAP level ( > 10 mcg/mL) were identified from our electronic health record. We manually screened charts for encounters involving APAP-opioid products and from these extracted demographic information, laboratory values, product(s) ingested, ingestion intent and amount, and disposition. We describe the number of encounters per year.
Results: Of 760 encounters, 186 (25%) involved APAP-opioid combination products. Most patients were Caucasian (83%), non-Hispanic (76%), and female (54%). The number of supratherapeutic APAP-opioid ingestions decreased over the 10-year period (Fig.1a). Most ingestions were acute (64%) and with intent at self-harm (57%). Most patients were discharged from the Emergency Department (37%) and not treated with N-acetylcysteine (65%). Only five cases developed acute liver failure. The most used combination product was hydrocodone/APAP (80%). A downtrend in hydrocodone/APAP accompanied a relative increase in codeine/APAP ingestions from 2015 onwards (Fig.1b).
Discussion: Our study shows that during the past 10 years the number of APAP-opioid supratherapeutic ingestions declined, especially hydrocodone/APAP likely at least in part due to its re-classification to schedule II. Codeine, schedule III, may have replaced some hydrocodone prescriptions.

Figure: Figure 1: Trends in acetaminophen and acetaminophen-opioid ingestions per year
a. Number of encounters per year of acetaminophen-only and acetaminophen-opioid supratherapeutic ingestions.
b. Number of encounters per year of each acetaminophen-opioid combination product.
a. Number of encounters per year of acetaminophen-only and acetaminophen-opioid supratherapeutic ingestions.
b. Number of encounters per year of each acetaminophen-opioid combination product.
Disclosures:
Elizabeth Harris indicated no relevant financial relationships.
Michael Harms indicated no relevant financial relationships.
Dazhe Cao indicated no relevant financial relationships.
Courtney Prestwood indicated no relevant financial relationships.
Lister DeBinya indicated no relevant financial relationships.
Kurt Kleinschmidt indicated no relevant financial relationships.
Amy Young indicated no relevant financial relationships.
Srishti Saha indicated no relevant financial relationships.
Jody Rule indicated no relevant financial relationships.
Kristin Alvarez indicated no relevant financial relationships.
Sandeep Das indicated no relevant financial relationships.
William Lee indicated no relevant financial relationships.
Elizabeth Harris, MD1, Michael Harms, MS2, Dazhe Cao, MD3, Courtney Prestwood, MD4, Lister DeBinya, BS1, Kurt Kleinschmidt, MD1, Amy Young, MD5, Srishti Saha, MD1, Jody Rule, PhD1, Kristin Alvarez, PharmD2, Sandeep R. Das, MD, MPH1, William Lee, MD1. C0525 - Federal Mandates and Trends in Acetaminophen-Opioid Combination Product Supratherapeutic Ingestions at a Large Urban Safety-Net Hospital From 2011-2020, ACG 2022 Annual Scientific Meeting Abstracts. Charlotte, NC: American College of Gastroenterology.
