Back


Poster Session C - Monday Afternoon
Category: Liver
C0542 - A Case of Labetalol-Induced Liver Injury
Monday, October 24, 2022
3:00 PM – 5:00 PM ET
Location: Crown Ballroom

Has Audio
- NS
Naveena Sunkara, MD
Brown University
Providence, RI
Presenting Author(s)
Naveena Sunkara, MD1, George M. Hanna, MD2, Simran Gupta, MD1, Nabil Toubia, MD3
1Brown University, Providence, RI; 2Warren Alpert Medical School of Brown University, Providence, RI; 3Roger Williams Medical Center, Providence, RI
Introduction: Drug induced liver injury (DILI) is a rare but important cause of hepatotoxicity. Presentation varies widely, from mild elevations of serum aminotransferases to fulminant hepatic failure. Here, we present a case of DILI secondary to labetalol use, a rarely cited cause.
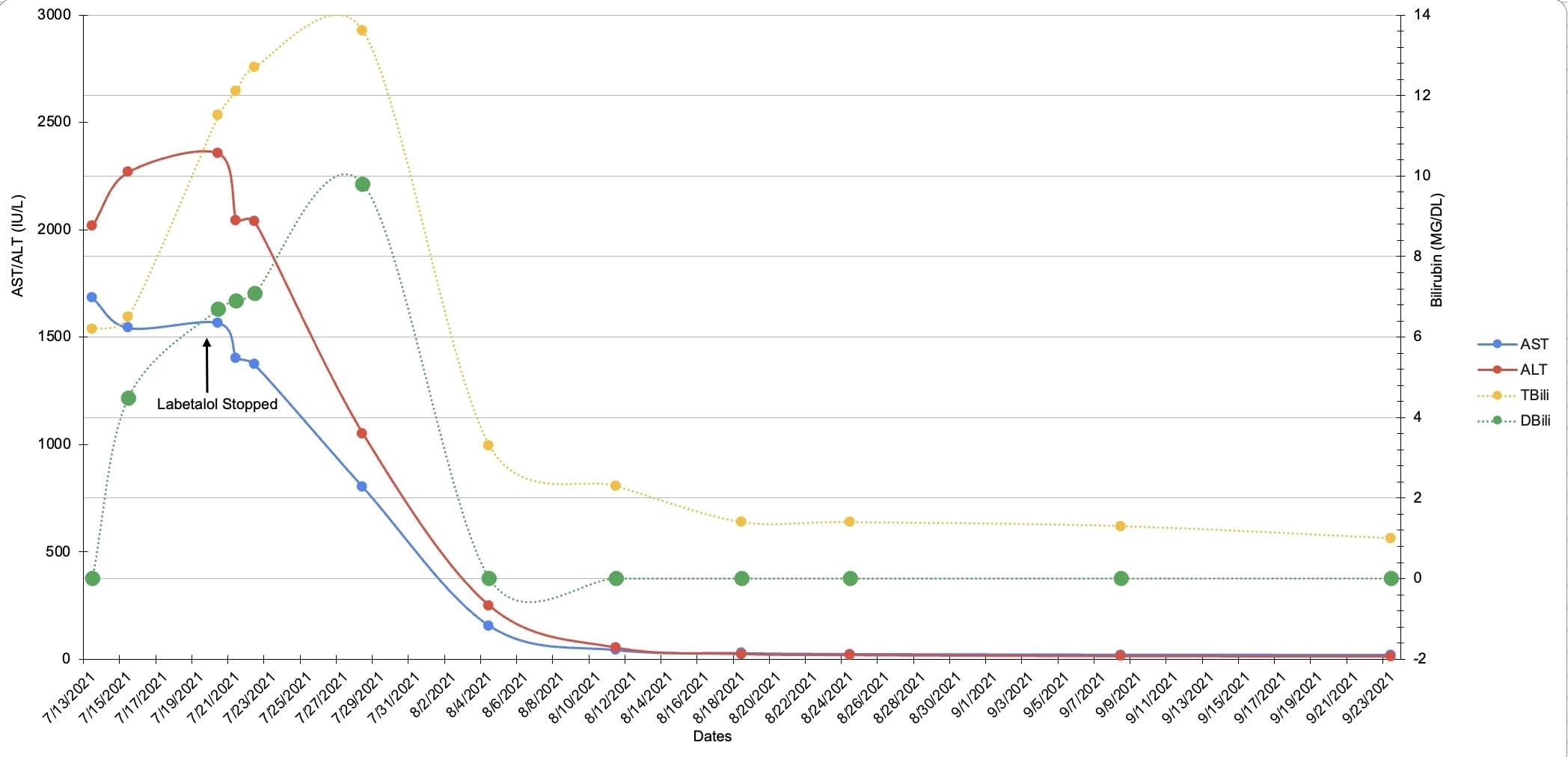
Case Description/Methods: A 36-year-old woman with a history of hypertension presented to the emergency room with 10 days of progressive painless jaundice. She switched from hydrochlorothiazide to labetalol 9 weeks prior to presentation. Vitals were within normal limits and her exam was remarkable for scleral icterus and jaundice of the skin. Laboratory investigation revealed AST 2,042 IU/L, ALT 1,402 IU/L, total bilirubin 12.1 mg/dL, direct bilirubin 6.9 mg/dL, ANA titer 1:160 and IgG 2,078 g/L. CT abdomen pelvis was unremarkable. Given clinical stability, the patient was discharged with instruction to discontinue labetalol use indefinitely. Her labs and clinical status were closely followed by outpatient GI. Her symptoms and lab abnormalities resolved within two months without further intervention (Figure 1) .
Discussion: Labetalol has been associated with mild-to-moderate elevations of serum aminotransferase levels in up to 8% of patients, a rate far higher than with other beta-blockers. DILI is a diagnosis of exclusion and may pose a diagnostic challenge, especially in the setting of drugs that are not commonly implicated. Our case is consistent with other reports that describe typical symptom onset between 5 to 90 days after initial exposure to a drug. Our patient first noted symptoms 53 days after labetalol initiation and resolution of symptoms and normalization of labs 66 days after cessation. Additional associated symptoms reported with labetalol mediated DILI include jaundice, abdominal pain, pruritus, nausea and dark urine. Autoantibody formation is rare in labetolol mediated toxicity, however, it is unclear in this case whether autoantibody formation was drug mediated or found incidentally. Our patient improved with drug cessation alone and did not require steroid therapy. Providers must take care to keep a broad differential for liver injury in the absence of a typical history, as a missed diagnosis of DILI without prompt intervention may progress to fulminant liver failure.
Disclosures:
Naveena Sunkara, MD1, George M. Hanna, MD2, Simran Gupta, MD1, Nabil Toubia, MD3. C0542 - A Case of Labetalol-Induced Liver Injury, ACG 2022 Annual Scientific Meeting Abstracts. Charlotte, NC: American College of Gastroenterology.
1Brown University, Providence, RI; 2Warren Alpert Medical School of Brown University, Providence, RI; 3Roger Williams Medical Center, Providence, RI
Introduction: Drug induced liver injury (DILI) is a rare but important cause of hepatotoxicity. Presentation varies widely, from mild elevations of serum aminotransferases to fulminant hepatic failure. Here, we present a case of DILI secondary to labetalol use, a rarely cited cause.
Case Description/Methods: A 36-year-old woman with a history of hypertension presented to the emergency room with 10 days of progressive painless jaundice. She switched from hydrochlorothiazide to labetalol 9 weeks prior to presentation. Vitals were within normal limits and her exam was remarkable for scleral icterus and jaundice of the skin. Laboratory investigation revealed AST 2,042 IU/L, ALT 1,402 IU/L, total bilirubin 12.1 mg/dL, direct bilirubin 6.9 mg/dL, ANA titer 1:160 and IgG 2,078 g/L. CT abdomen pelvis was unremarkable. Given clinical stability, the patient was discharged with instruction to discontinue labetalol use indefinitely. Her labs and clinical status were closely followed by outpatient GI. Her symptoms and lab abnormalities resolved within two months without further intervention (Figure 1) .
Discussion: Labetalol has been associated with mild-to-moderate elevations of serum aminotransferase levels in up to 8% of patients, a rate far higher than with other beta-blockers. DILI is a diagnosis of exclusion and may pose a diagnostic challenge, especially in the setting of drugs that are not commonly implicated. Our case is consistent with other reports that describe typical symptom onset between 5 to 90 days after initial exposure to a drug. Our patient first noted symptoms 53 days after labetalol initiation and resolution of symptoms and normalization of labs 66 days after cessation. Additional associated symptoms reported with labetalol mediated DILI include jaundice, abdominal pain, pruritus, nausea and dark urine. Autoantibody formation is rare in labetolol mediated toxicity, however, it is unclear in this case whether autoantibody formation was drug mediated or found incidentally. Our patient improved with drug cessation alone and did not require steroid therapy. Providers must take care to keep a broad differential for liver injury in the absence of a typical history, as a missed diagnosis of DILI without prompt intervention may progress to fulminant liver failure.

Figure: Figure 1: Trend of AST, ALT, Direct and Total Bilirubin after labetalol cessation
Disclosures:
Naveena Sunkara indicated no relevant financial relationships.
George Hanna indicated no relevant financial relationships.
Simran Gupta indicated no relevant financial relationships.
Nabil Toubia indicated no relevant financial relationships.
Naveena Sunkara, MD1, George M. Hanna, MD2, Simran Gupta, MD1, Nabil Toubia, MD3. C0542 - A Case of Labetalol-Induced Liver Injury, ACG 2022 Annual Scientific Meeting Abstracts. Charlotte, NC: American College of Gastroenterology.
