Back

Poster Session A - Sunday Afternoon
Category: Obesity
A0594 - Generation of a High-Affinity, Humanized Monoclonal Antibody (mAb) That Effectively Neutralizes Glucose-Dependent Insulinotropic Polypeptide (GIP)
Sunday, October 23, 2022
5:00 PM – 7:00 PM ET
Location: Crown Ballroom

Has Audio
- MB
Michael O. Boylan, PhD
9 Meters
Raleigh, NC
Presenting Author(s)
Michael O. Boylan, PhD1, Patrick H. Griffin, MD1, Sireesh Appajosyula, PharmD1, Michael Wolfe, MD, FACG2
19 Meters, Raleigh, NC; 2Case Western Reserve University, Bal Harbour, FL
Introduction: GIP, a peptide hormone synthesized in intestinal K-cells, is both insulinotropic and insulin mimetic and accordingly plays a critical role in promoting nutrient uptake and storage. We recently developed a mouse mAb (mmAb) and reported that this mmAb decreased weight gain in C57BL/6 mice fed a high-fat diet by nearly 50%, without affecting food intake. The aim of this study was to “humanize” our GIP mmAb by grafting its complementary determining regions onto a human IgG “scaffold” and to characterize its binding characteristics.
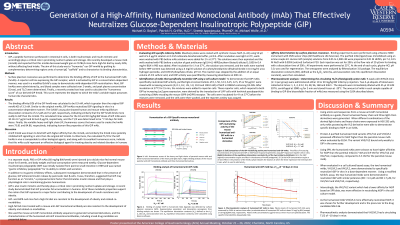
Methods: Surface plasmon resonance was performed to determine the binding affinity of GIP to the humanized GIP mAb (hmAb). A reporter cell line expressing the GIP receptor, which is activated by GIP in a concentration-dependent manner, was used in a modified Schild’s assay to demonstrate mAb-dependent GIP neutralization. Next, GIP hmAbs were administered ip, and blood samples were collected over 2 weeks, and peak plasma concentration (Cmax), and T1/2 were determined. Finally, a recently created tool was used to calculate the “humanness score” of our derived GIP hmAb. This score represents the degree to which the mAb’s variable region possesses human-like characteristics.
Results: The binding affinity (KD) of the GIP hmAb was calculated to be 0.9 nM, which is greater than the original GIP mmAb KD of 3.3 nM. Similar to the original mmAb, GIP hmAbs neutralized GIP signaling in vitro in a concentration-dependent manner. The Schild’s assay plot showed human and mouse mAb equilibrium dissociation constants of 2.2 µM and 3.2 µM, respectively, indicating similarly that the GIP hmAb binds more avidly to GIP than the mmAb. The calculated Cmax values for the 10 and 30 mg/kg BW doses of GIP mAb were 10.12± 0.7 μg/ml and 32.6±1.0 μg/ml, respectively, and the T1/2 was determined to be ~7-10 days for both doses. Finally, the variable heavy and light chain (VL) humanness scores that were used to create the hmAb were 75.81 and 84.86, respectively, indicating human-like properties of this GIP mAb.
Discussion: A GIP hmAb was shown to bind GIP with higher affinity than the mmAb, and similarly the hmAb more potently inhibited GIP signaling in vitro than the original GIP mmAb. Furthermore, the calculated T1/2 for the GIP hmAb in vivo is comparable to other biological agents, and along with its excellent humanness score, indicate that this mAb could represent an effective biological agent for treating obesity and related disorders in humans.
Disclosures:
Michael O. Boylan, PhD1, Patrick H. Griffin, MD1, Sireesh Appajosyula, PharmD1, Michael Wolfe, MD, FACG2. A0594 - Generation of a High-Affinity, Humanized Monoclonal Antibody (mAb) That Effectively Neutralizes Glucose-Dependent Insulinotropic Polypeptide (GIP), ACG 2022 Annual Scientific Meeting Abstracts. Charlotte, NC: American College of Gastroenterology.
19 Meters, Raleigh, NC; 2Case Western Reserve University, Bal Harbour, FL
Introduction: GIP, a peptide hormone synthesized in intestinal K-cells, is both insulinotropic and insulin mimetic and accordingly plays a critical role in promoting nutrient uptake and storage. We recently developed a mouse mAb (mmAb) and reported that this mmAb decreased weight gain in C57BL/6 mice fed a high-fat diet by nearly 50%, without affecting food intake. The aim of this study was to “humanize” our GIP mmAb by grafting its complementary determining regions onto a human IgG “scaffold” and to characterize its binding characteristics.
Methods: Surface plasmon resonance was performed to determine the binding affinity of GIP to the humanized GIP mAb (hmAb). A reporter cell line expressing the GIP receptor, which is activated by GIP in a concentration-dependent manner, was used in a modified Schild’s assay to demonstrate mAb-dependent GIP neutralization. Next, GIP hmAbs were administered ip, and blood samples were collected over 2 weeks, and peak plasma concentration (Cmax), and T1/2 were determined. Finally, a recently created tool was used to calculate the “humanness score” of our derived GIP hmAb. This score represents the degree to which the mAb’s variable region possesses human-like characteristics.
Results: The binding affinity (KD) of the GIP hmAb was calculated to be 0.9 nM, which is greater than the original GIP mmAb KD of 3.3 nM. Similar to the original mmAb, GIP hmAbs neutralized GIP signaling in vitro in a concentration-dependent manner. The Schild’s assay plot showed human and mouse mAb equilibrium dissociation constants of 2.2 µM and 3.2 µM, respectively, indicating similarly that the GIP hmAb binds more avidly to GIP than the mmAb. The calculated Cmax values for the 10 and 30 mg/kg BW doses of GIP mAb were 10.12± 0.7 μg/ml and 32.6±1.0 μg/ml, respectively, and the T1/2 was determined to be ~7-10 days for both doses. Finally, the variable heavy and light chain (VL) humanness scores that were used to create the hmAb were 75.81 and 84.86, respectively, indicating human-like properties of this GIP mAb.
Discussion: A GIP hmAb was shown to bind GIP with higher affinity than the mmAb, and similarly the hmAb more potently inhibited GIP signaling in vitro than the original GIP mmAb. Furthermore, the calculated T1/2 for the GIP hmAb in vivo is comparable to other biological agents, and along with its excellent humanness score, indicate that this mAb could represent an effective biological agent for treating obesity and related disorders in humans.
Disclosures:
Michael Boylan: 9 Meters – Consultant. Lobesity LLC – Employee.
Patrick Griffin: 9 Meters – Employee, Stock-publicly held company(excluding mutual/index funds).
Sireesh Appajosyula: 9 Meters – Employee, Stock-publicly held company(excluding mutual/index funds).
Michael Wolfe: 9 Meters – Consultant. Lobesity LLC – Employee.
Michael O. Boylan, PhD1, Patrick H. Griffin, MD1, Sireesh Appajosyula, PharmD1, Michael Wolfe, MD, FACG2. A0594 - Generation of a High-Affinity, Humanized Monoclonal Antibody (mAb) That Effectively Neutralizes Glucose-Dependent Insulinotropic Polypeptide (GIP), ACG 2022 Annual Scientific Meeting Abstracts. Charlotte, NC: American College of Gastroenterology.
