Back

Poster Session C - Monday Afternoon
Category: Endoscopy Video Forum
C0200 - Chronic Tracheoesophageal Fistula Successfully Treated Using an Amplatzer Closure Device Under Endoscopic Visualization
Monday, October 24, 2022
3:00 PM – 5:00 PM ET
Location: Crown Ballroom

Has Audio

Karolina N. Dziadkowiec, MD
University of Texas Health San Antonio
San Antonio, TX
Presenting Author(s)
Karolina N. Dziadkowiec, MD, Peter M. Stawinski, MD, Nichole Henkes, , Natasha McMillan, MD, Laura Rosenkranz, MD
University of Texas Health San Antonio, San Antonio, TX
Introduction: In tertiary care centers, where advanced endoscopic expertise is available, the placement of an Amplatzer septal occluder is practical and safe. This allows for potential fistula closure, especially in patients with severe comorbidities or in critical condition or those with chronic or recurrent tracheoesophageal fistulas (TEF).
Case Description/Methods: A 72-year-old man with a history of gastroesophageal reflux disease (GERD) and well-differentiated esophageal adenocarcinoma who presented for dysphagia and cough. Social history was negative for smoking, smokeless tobacco, alcohol ingestion and illicit drug use. Home medications include famotidine. He had no known significant family history.
The patient had undergone numerous esophagogastroduodenoscopies (EGD) since 2017 for recurrent strictures requiring TTS balloon dilations, intralesional triamcinolone acetonide injections and esophageal stenting complicated by formation of a tracheoesophageal fistula (TEF) at the anastomotic site. The TEF was initially treated with stenting 3 months prior to presentation.
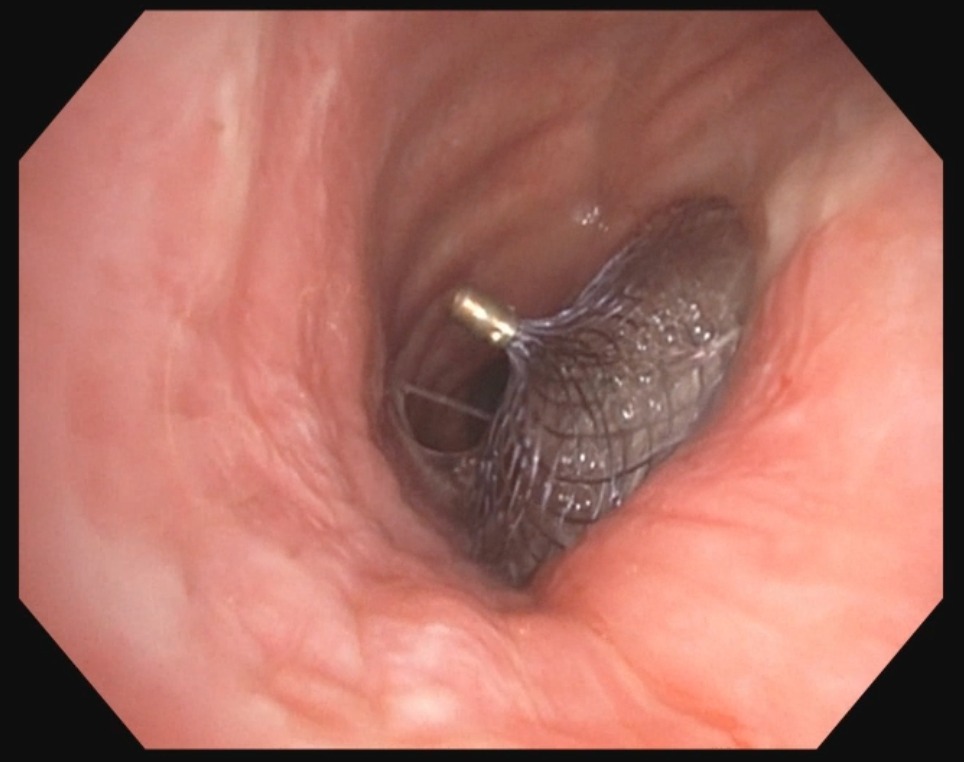
The patient underwent EGD and bronchoscopy with visualization of a 5 mm fistula. He was then evaluated by the surgery team for consideration of surgical repair, however the patient declined surgical intervention. Given the clinical condition of the patient with recurrent aspiration events, failed endoscopic management and refusal of surgical intervention, it was decided to remove the esophageal stent and attempt placement of an Amplatzer septal occluder. An 18 mm Amplatzer device (AD) was successfully placed with satisfactory closure of the TEF. Repeat endoscopy 5 months later, showed that the device had remained in the correct position and the patient remained asymptomatic following advancement of his diet.
Discussion: Acquired TEF is a rare complication, the incidence in the US has yet to be reported. Amplatzer occluder devices, originally designed for transcatheter closure of cardiac defects, have shown promise in the treatment of TEFs. It is suggested that the placement of the AD induces granulation tissue formation around the device allowing for complete closure of the fistula without compromising airway patency. Upon review of current literature only one other case report was found where an AD was used for the management of refractory TEF. In the setting of large or difficult to manage TEFs and patients who are not surgical candidates, Amplatzer septal occluder devices can be used to successfully treat complicated TEFs.
Disclosures:
Karolina N. Dziadkowiec, MD, Peter M. Stawinski, MD, Nichole Henkes, , Natasha McMillan, MD, Laura Rosenkranz, MD. C0200 - Chronic Tracheoesophageal Fistula Successfully Treated Using an Amplatzer Closure Device Under Endoscopic Visualization, ACG 2022 Annual Scientific Meeting Abstracts. Charlotte, NC: American College of Gastroenterology.
University of Texas Health San Antonio, San Antonio, TX
Introduction: In tertiary care centers, where advanced endoscopic expertise is available, the placement of an Amplatzer septal occluder is practical and safe. This allows for potential fistula closure, especially in patients with severe comorbidities or in critical condition or those with chronic or recurrent tracheoesophageal fistulas (TEF).
Case Description/Methods: A 72-year-old man with a history of gastroesophageal reflux disease (GERD) and well-differentiated esophageal adenocarcinoma who presented for dysphagia and cough. Social history was negative for smoking, smokeless tobacco, alcohol ingestion and illicit drug use. Home medications include famotidine. He had no known significant family history.
The patient had undergone numerous esophagogastroduodenoscopies (EGD) since 2017 for recurrent strictures requiring TTS balloon dilations, intralesional triamcinolone acetonide injections and esophageal stenting complicated by formation of a tracheoesophageal fistula (TEF) at the anastomotic site. The TEF was initially treated with stenting 3 months prior to presentation.
The patient underwent EGD and bronchoscopy with visualization of a 5 mm fistula. He was then evaluated by the surgery team for consideration of surgical repair, however the patient declined surgical intervention. Given the clinical condition of the patient with recurrent aspiration events, failed endoscopic management and refusal of surgical intervention, it was decided to remove the esophageal stent and attempt placement of an Amplatzer septal occluder. An 18 mm Amplatzer device (AD) was successfully placed with satisfactory closure of the TEF. Repeat endoscopy 5 months later, showed that the device had remained in the correct position and the patient remained asymptomatic following advancement of his diet.
Discussion: Acquired TEF is a rare complication, the incidence in the US has yet to be reported. Amplatzer occluder devices, originally designed for transcatheter closure of cardiac defects, have shown promise in the treatment of TEFs. It is suggested that the placement of the AD induces granulation tissue formation around the device allowing for complete closure of the fistula without compromising airway patency. Upon review of current literature only one other case report was found where an AD was used for the management of refractory TEF. In the setting of large or difficult to manage TEFs and patients who are not surgical candidates, Amplatzer septal occluder devices can be used to successfully treat complicated TEFs.

Figure: Amplatzer device deployed with successful tracheoesophageal fistula closure.
Disclosures:
Karolina Dziadkowiec indicated no relevant financial relationships.
Peter Stawinski indicated no relevant financial relationships.
Nichole Henkes indicated no relevant financial relationships.
Natasha McMillan indicated no relevant financial relationships.
Laura Rosenkranz indicated no relevant financial relationships.
Karolina N. Dziadkowiec, MD, Peter M. Stawinski, MD, Nichole Henkes, , Natasha McMillan, MD, Laura Rosenkranz, MD. C0200 - Chronic Tracheoesophageal Fistula Successfully Treated Using an Amplatzer Closure Device Under Endoscopic Visualization, ACG 2022 Annual Scientific Meeting Abstracts. Charlotte, NC: American College of Gastroenterology.

