Back

Poster Session B - Monday Morning
Category: IBD
B0431 - New-Onset Crohn’s Disease Unmasked by Secukinumab
Monday, October 24, 2022
10:00 AM – 12:00 PM ET
Location: Crown Ballroom

Has Audio

Anas Khouri, MD
University of South Alabama
Mobile, AL
Presenting Author(s)
Anas Khouri, MD, Cesar Moreno, MD, Benjamin Niland, MD
University of South Alabama, Mobile, AL
Introduction: Psoriasis is an autoimmune disease that affects 1% to 2% of the population. Patients with this condition showed high expression of proinflammatory cytokines including interleukin (IL)-17. Secukinumab, an IL-17 inhibitor, is indicated for the treatment of psoriasis and psoriatic arthritis. IL-17 inhibition in patients with Crohn’s disease may be associated with worsening of the disease. We present the case of a 38-year-old female who received secukinumab therapy for psoriatic arthritis with subsequent unmasking of Crohn’s disease.
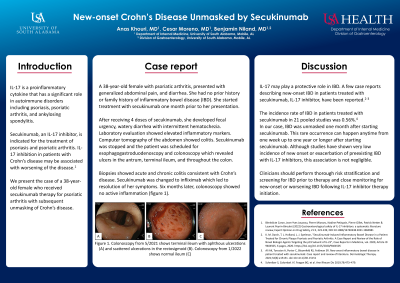
Case Description/Methods: A 38-year-old female with psoriatic arthritis, presented with generalized abdominal pain, and diarrhea. She had no prior history or family history of inflammatory bowel disease (IBD). She started treatment with secukinumab one month prior to her presentation. After receiving 4 doses of secukinumab, she developed fecal urgency, watery diarrhea with intermittent hematochezia. Laboratory evaluation showed elevated inflammatory markers. Computed tomography of the abdomen showed colitis. Secukinumab was stopped and the patient was scheduled for esophagogastroduodenoscopy and colonoscopy which revealed ulcers in the antrum, terminal ileum, and throughout the colon. Biopsies showed acute and chronic colitis consistent with Crohn’s disease. Secukinumab was changed to infliximab which led to resolution of her symptoms. Six months later, colonoscopy showed no active inflammation (figure 1).
Discussion: IL-17 is a proinflammatory cytokine that has a significant role in autoimmune disorders including psoriasis and psoriatic arthritis. IL-17 may play a protective role in IBD. A few case reports describing new-onset IBD in patients treated with secukinumab, IL-17 inhibitor, have been reported. The incidence rate of IBD in patients treated with secukinumab in 21 pooled studies was 0.56%.
In our case, IBD was unmasked one month after starting secukinumab. This rare occurrence can happen anytime from one week up to one year or longer after starting secukinumab. Although studies have shown very low incidence of new onset or exacerbation of preexisting IBD with IL-17 inhibitors, this association is not negligible. Clinicians should perform thorough risk stratification and screening for IBD prior to therapy and close monitoring for new-onset or worsening IBD following IL-17 inhibitor therapy initiation. Other agents that target IL-23 (ustekinumab) instead of IL-17 have shown promise in treating both psoriasis and IBD.

Disclosures:
Anas Khouri, MD, Cesar Moreno, MD, Benjamin Niland, MD. B0431 - New-Onset Crohn’s Disease Unmasked by Secukinumab, ACG 2022 Annual Scientific Meeting Abstracts. Charlotte, NC: American College of Gastroenterology.
University of South Alabama, Mobile, AL
Introduction: Psoriasis is an autoimmune disease that affects 1% to 2% of the population. Patients with this condition showed high expression of proinflammatory cytokines including interleukin (IL)-17. Secukinumab, an IL-17 inhibitor, is indicated for the treatment of psoriasis and psoriatic arthritis. IL-17 inhibition in patients with Crohn’s disease may be associated with worsening of the disease. We present the case of a 38-year-old female who received secukinumab therapy for psoriatic arthritis with subsequent unmasking of Crohn’s disease.
Case Description/Methods: A 38-year-old female with psoriatic arthritis, presented with generalized abdominal pain, and diarrhea. She had no prior history or family history of inflammatory bowel disease (IBD). She started treatment with secukinumab one month prior to her presentation. After receiving 4 doses of secukinumab, she developed fecal urgency, watery diarrhea with intermittent hematochezia. Laboratory evaluation showed elevated inflammatory markers. Computed tomography of the abdomen showed colitis. Secukinumab was stopped and the patient was scheduled for esophagogastroduodenoscopy and colonoscopy which revealed ulcers in the antrum, terminal ileum, and throughout the colon. Biopsies showed acute and chronic colitis consistent with Crohn’s disease. Secukinumab was changed to infliximab which led to resolution of her symptoms. Six months later, colonoscopy showed no active inflammation (figure 1).
Discussion: IL-17 is a proinflammatory cytokine that has a significant role in autoimmune disorders including psoriasis and psoriatic arthritis. IL-17 may play a protective role in IBD. A few case reports describing new-onset IBD in patients treated with secukinumab, IL-17 inhibitor, have been reported. The incidence rate of IBD in patients treated with secukinumab in 21 pooled studies was 0.56%.
In our case, IBD was unmasked one month after starting secukinumab. This rare occurrence can happen anytime from one week up to one year or longer after starting secukinumab. Although studies have shown very low incidence of new onset or exacerbation of preexisting IBD with IL-17 inhibitors, this association is not negligible. Clinicians should perform thorough risk stratification and screening for IBD prior to therapy and close monitoring for new-onset or worsening IBD following IL-17 inhibitor therapy initiation. Other agents that target IL-23 (ustekinumab) instead of IL-17 have shown promise in treating both psoriasis and IBD.

Figure: Figure 1. Colonoscopy from 5/2021 shows terminal ileum with aphthous ulcerations (A) and scattered ulcerations in the rectosigmoid (B). Colonoscopy from 1/2022 shows normal ileum (C)
Disclosures:
Anas Khouri indicated no relevant financial relationships.
Cesar Moreno indicated no relevant financial relationships.
Benjamin Niland indicated no relevant financial relationships.
Anas Khouri, MD, Cesar Moreno, MD, Benjamin Niland, MD. B0431 - New-Onset Crohn’s Disease Unmasked by Secukinumab, ACG 2022 Annual Scientific Meeting Abstracts. Charlotte, NC: American College of Gastroenterology.
