Back

Poster Session B - Monday Morning
Category: IBD
B0386 - Real World Clinical Effectiveness and Safety of Vedolizumab and Adalimumab in Biologic-Naïve Patients With Crohn’s Disease: Results From the EVOLVE Study
Monday, October 24, 2022
10:00 AM – 12:00 PM ET
Location: Crown Ballroom

Has Audio

Andres Yarur, MD
Cedars-Sinai Medical Center
Los Angeles, CA
Presenting Author(s)
Andres Yarur, MD1, Brian Bressler, MD, MS2, Neil Brett, PhD3, Marielle Bassel, PhD3, Shashi Adsul, MD4, Pravin Kamble, PhD5, Gerassimos J. Mantzaris, MD, PhD6
1Cedars-Sinai Medical Center, Los Angeles, CA; 2University of British Columbia, Vancouver, BC, Canada; 3PPD, part of Thermo Fisher Scientific, Montreal, PQ, Canada; 4Takeda Pharmaceuticals International AG, Zurich, Zurich, Switzerland; 5Takeda Pharmaceuticals International, Cambridge, MA; 6Evangelismos-Polykliniki’ General Hospital, Athens, Attiki, Greece
Introduction: Vedolizumab (VDZ, a gut selective anti-α4β7-integrin) and adalimumab (ADA, anti-tumor necrosis factor) are approved biologic treatments for moderate to severe Crohn’s disease (CD). We aimed to evaluate the real-world clinical effectiveness and safety of VDZ versus ADA in biologic-naïve patients with CD.
Methods: This was a retrospective cohort study in adult patients with CD who received first-line biologic treatment with VDZ or ADA at 37 sites in the US, Canada, and Greece. The index date was defined as the date of first-line biologic initiation between May 2014 and March 2018. Cumulative rates of treatment persistence and clinical outcomes over 12 months were estimated using the Kaplan-Meier method. Patients were censored at loss of follow up, death, end of chart abstraction, treatment discontinuation, or dose escalation, whichever came first. Clinical outcomes were assessed using pre-defined hierarchical algorithms of standard measures.1 Survival analyses were conducted for safety outcomes, CD-related exacerbations and CD surgeries. Adjusted analyses were performed using stabilized inverse probability weighting using baseline covariates.
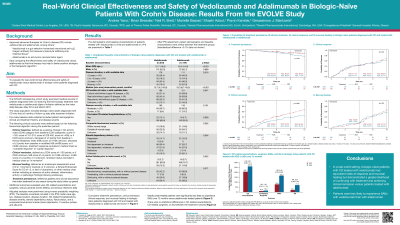
Results: 362 patients were included (VDZ: 218; ADA: 144). Baseline characteristics are shown in Table 1. Cumulative rates of clinical response (68.5% vs 61.1%; p=0.59) and mucosal healing (67.7% vs 56.0%; p=0.56) over 12 months were similar between treatment cohorts, whilst clinical remission rates (66.3% vs 46.4%; p< 0.01) were greater in VDZ- versus ADA-treated patients. Over 12 months, VDZ-treated patients were more likely to persist on treatment versus ADA-treated patients (89.3% vs 77.5%; p=0.02). VDZ-treated patients were significantly less likely to experience (incidence rates per 1000 person-years; HR [95%CI]) serious adverse events within 1 year (78.9 vs 166.2; 0.45 [0.22-0.93]), but there were no statistical differences in CD exacerbations (207.7 vs 261.8; 0.91 [0.56-1.47]), CD-related surgeries (10.3 vs 16.0; 1.55 [0.21-11.15]) or serious infections (20.5 vs 71.2; 0.27 [0.06-1.20]) versus ADA-treated patients.
Discussion: In a real-world setting in biologic-naïve patients with CD, VDZ-treated patients had equivalent rates of response and mucosal healing but a greater likelihood of persisting on treatment and achieving clinical remission versus ADA-treated patients. Studies assessing outcomes after dose optimization are needed.
1Bressler, B. et al. J Crohns Colitis. 2021 Oct 7;15(10):1694-1706.
Disclosures:
Andres Yarur, MD1, Brian Bressler, MD, MS2, Neil Brett, PhD3, Marielle Bassel, PhD3, Shashi Adsul, MD4, Pravin Kamble, PhD5, Gerassimos J. Mantzaris, MD, PhD6. B0386 - Real World Clinical Effectiveness and Safety of Vedolizumab and Adalimumab in Biologic-Naïve Patients With Crohn’s Disease: Results From the EVOLVE Study, ACG 2022 Annual Scientific Meeting Abstracts. Charlotte, NC: American College of Gastroenterology.
1Cedars-Sinai Medical Center, Los Angeles, CA; 2University of British Columbia, Vancouver, BC, Canada; 3PPD, part of Thermo Fisher Scientific, Montreal, PQ, Canada; 4Takeda Pharmaceuticals International AG, Zurich, Zurich, Switzerland; 5Takeda Pharmaceuticals International, Cambridge, MA; 6Evangelismos-Polykliniki’ General Hospital, Athens, Attiki, Greece
Introduction: Vedolizumab (VDZ, a gut selective anti-α4β7-integrin) and adalimumab (ADA, anti-tumor necrosis factor) are approved biologic treatments for moderate to severe Crohn’s disease (CD). We aimed to evaluate the real-world clinical effectiveness and safety of VDZ versus ADA in biologic-naïve patients with CD.
Methods: This was a retrospective cohort study in adult patients with CD who received first-line biologic treatment with VDZ or ADA at 37 sites in the US, Canada, and Greece. The index date was defined as the date of first-line biologic initiation between May 2014 and March 2018. Cumulative rates of treatment persistence and clinical outcomes over 12 months were estimated using the Kaplan-Meier method. Patients were censored at loss of follow up, death, end of chart abstraction, treatment discontinuation, or dose escalation, whichever came first. Clinical outcomes were assessed using pre-defined hierarchical algorithms of standard measures.1 Survival analyses were conducted for safety outcomes, CD-related exacerbations and CD surgeries. Adjusted analyses were performed using stabilized inverse probability weighting using baseline covariates.
Results: 362 patients were included (VDZ: 218; ADA: 144). Baseline characteristics are shown in Table 1. Cumulative rates of clinical response (68.5% vs 61.1%; p=0.59) and mucosal healing (67.7% vs 56.0%; p=0.56) over 12 months were similar between treatment cohorts, whilst clinical remission rates (66.3% vs 46.4%; p< 0.01) were greater in VDZ- versus ADA-treated patients. Over 12 months, VDZ-treated patients were more likely to persist on treatment versus ADA-treated patients (89.3% vs 77.5%; p=0.02). VDZ-treated patients were significantly less likely to experience (incidence rates per 1000 person-years; HR [95%CI]) serious adverse events within 1 year (78.9 vs 166.2; 0.45 [0.22-0.93]), but there were no statistical differences in CD exacerbations (207.7 vs 261.8; 0.91 [0.56-1.47]), CD-related surgeries (10.3 vs 16.0; 1.55 [0.21-11.15]) or serious infections (20.5 vs 71.2; 0.27 [0.06-1.20]) versus ADA-treated patients.
Discussion: In a real-world setting in biologic-naïve patients with CD, VDZ-treated patients had equivalent rates of response and mucosal healing but a greater likelihood of persisting on treatment and achieving clinical remission versus ADA-treated patients. Studies assessing outcomes after dose optimization are needed.
1Bressler, B. et al. J Crohns Colitis. 2021 Oct 7;15(10):1694-1706.
Table: Abbreviations: GI = gastrointestinal; SD = standard deviation. *While all patients were required to have 6 months follow-up from time of treatment initiation to data abstraction, some patients were lost to follow-up and therefore minimum duration during the observation period was <6 months.
Disclosures:
Andres Yarur: Arena Pharmaceuticals – Consultant. Bristol Myers Squibb – Consultant. Prometheus Laboratories – Consultant. Takeda – Advisory Committee/Board Member.
Brian Bressler: Abbvie – Advisor or Review Panel Member, Grant/Research Support, Speakers Bureau. Allergan – Advisor or Review Panel Member. Alvine – Grant/Research Support. Amgen – Advisor or Review Panel Member, Grant/Research Support. Boehringer Ingelheim – Grant/Research Support. Bristol Myers Squibb – Advisor or Review Panel Member, Grant/Research Support. Celgene – Advisor or Review Panel Member, Grant/Research Support. Ferring – Advisor or Review Panel Member, Speakers Bureau. Genentech – Advisor or Review Panel Member, Grant/Research Support. GSK – Grant/Research Support. Janssen – Advisor or Review Panel Member, Grant/Research Support, Speakers Bureau. Merck – Advisor or Review Panel Member, Grant/Research Support, Speakers Bureau. Microbiome Insights – Advisor or Review Panel Member. Novartis – Advisor or Review Panel Member, Speakers Bureau. Pendopharm – Advisor or Review Panel Member. Pfizer – Advisor or Review Panel Member, Speakers Bureau. Protagonist – Advisor or Review Panel Member. Qu Biologic – Grant/Research Support, Stock Options. Robarts Clinical Trials – Advisor or Review Panel Member. Takeda – Advisor or Review Panel Member, Speakers Bureau.
Neil Brett: Evidera – Employee. Takeda – Funding.
Marielle Bassel: Evidera – Employee. Takeda – Funding.
Shashi Adsul: Takeda – Employee, Stock Options, Stock-publicly held company(excluding mutual/index funds).
Pravin Kamble: Takeda – Employee, Stock Options, Stock-publicly held company(excluding mutual/index funds).
Gerassimos Mantzaris: AbbVie – Advisor or Review Panel Member, Grant/Research Support. Aenorasis – Advisor or Review Panel Member. Angelini – Advisor or Review Panel Member. Celgene – Advisor or Review Panel Member. Celltrion – Advisor or Review Panel Member. Dr Falk Pharma – Advisor or Review Panel Member. Ferring – Advisor or Review Panel Member, Grant/Research Support. Galenica – Grant/Research Support. Genesis – Grant/Research Support. Hospira – Advisor or Review Panel Member. Janssen – Advisor or Review Panel Member. Merck Sharp & Dohme – Advisor or Review Panel Member, Grant/Research Support. MYLAN – Advisor or Review Panel Member, Grant/Research Support. Pfizer – Advisor or Review Panel Member. Takeda – Advisor or Review Panel Member, Grant/Research Support. Vianex – Advisor or Review Panel Member, Grant/Research Support.
Andres Yarur, MD1, Brian Bressler, MD, MS2, Neil Brett, PhD3, Marielle Bassel, PhD3, Shashi Adsul, MD4, Pravin Kamble, PhD5, Gerassimos J. Mantzaris, MD, PhD6. B0386 - Real World Clinical Effectiveness and Safety of Vedolizumab and Adalimumab in Biologic-Naïve Patients With Crohn’s Disease: Results From the EVOLVE Study, ACG 2022 Annual Scientific Meeting Abstracts. Charlotte, NC: American College of Gastroenterology.
