Back

Poster Session C - Monday Afternoon
Category: Stomach
C0727 - Elucidating with Endoscopy: Investigating Surprising Symptoms in a Patient With a Gastric Electrical Stimulator
Monday, October 24, 2022
3:00 PM – 5:00 PM ET
Location: Crown Ballroom

Has Audio
- CM
Christo Mathew, MD
Baylor College of Medicine
Houston, TX
Presenting Author(s)
Christo Mathew, MD, Peter Nguyen, MD, Patrick Harris, MD, Reena Chokshi, MD
Baylor College of Medicine, Houston, TX
Introduction: Gastric electrical stimulators (GES) are long-term options for patients with gastroparesis refractory to pharmacotherapy and lifestyle changes. As GES use rises, recognizing device complications is crucial for managing patients with complex disease. This scenario highlights a case in which the etiology of the patient’s complications was discovered during endoscopy.
Case Description/Methods: A 30-year-old female with gastroparesis related to type 1 diabetes mellitus, and GES placement one year prior, was admitted for electrical shock sensations near her stimulator site, with radiation to her left arm and neck. It occurred about twelve times a day, and was associated with nausea and vomiting. She reported mild abdominal distension and feeling as if her pacer moved. There were no complications during device placement, and follow-up interrogations revealed no abnormalities. The patient denied other gastrointestinal symptoms or trauma to the pacer site area.
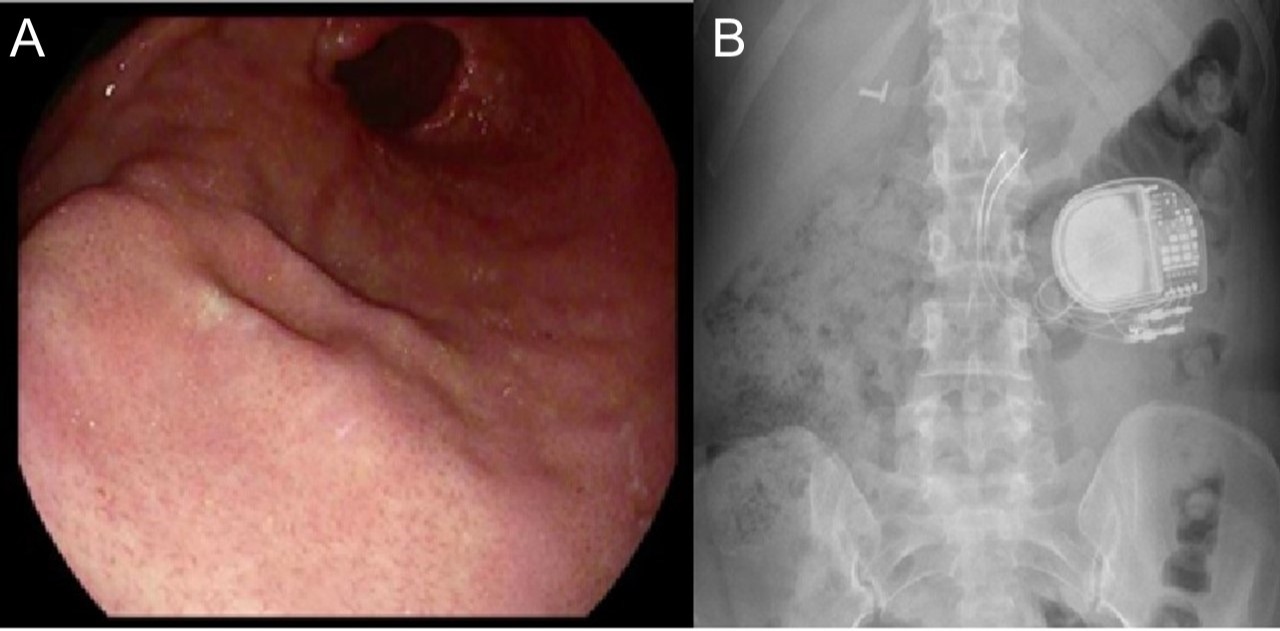
Her abdomen was soft with mild generalized tenderness, and the surgical scars from GES placement were well-healed. Device interrogation revealed settings at 2.5 volts, and impedance at 503 ohms. AP and lateral abdominal X-ray revealed proper lead placement. The decision was made to shut off the device, which ceased the shock sensations. Upper endoscopy revealed linear, subepithelial protrusions along the greater curvature of the antrum, located approximately 10.0 cm from the pylorus, consistent with typical gastric stimulator leads (Figure 1). After surgical consultation, stimulator leads were replaced, and the patient was discharged without continued symptoms.
Discussion: Adverse effects related to GES use include disruption of lead placement, erosion in the submucosa or mucosa, and obstruction, with generator site infection as the most common issue. Studies have shown that lead penetration into the gastric lumen occurs in 3% of patients, with 16% of patients requiring a corrective surgical procedure. This patient’s presentation demonstrates a rare event after GES placement. Typically, leads are placed in the muscularis propria of the greater gastric curvature. Lead displacement can be detected either on imaging or if impedance values are outside the normal range; however, neither of those occurred in this patient. Abnormal presentations demonstrated by patients with GES should be evaluated thoroughly, with suspicion for hardware malposition. Diagnostic work-up with endoscopy can guide further management.
Disclosures:
Christo Mathew, MD, Peter Nguyen, MD, Patrick Harris, MD, Reena Chokshi, MD. C0727 - Elucidating with Endoscopy: Investigating Surprising Symptoms in a Patient With a Gastric Electrical Stimulator, ACG 2022 Annual Scientific Meeting Abstracts. Charlotte, NC: American College of Gastroenterology.
Baylor College of Medicine, Houston, TX
Introduction: Gastric electrical stimulators (GES) are long-term options for patients with gastroparesis refractory to pharmacotherapy and lifestyle changes. As GES use rises, recognizing device complications is crucial for managing patients with complex disease. This scenario highlights a case in which the etiology of the patient’s complications was discovered during endoscopy.
Case Description/Methods: A 30-year-old female with gastroparesis related to type 1 diabetes mellitus, and GES placement one year prior, was admitted for electrical shock sensations near her stimulator site, with radiation to her left arm and neck. It occurred about twelve times a day, and was associated with nausea and vomiting. She reported mild abdominal distension and feeling as if her pacer moved. There were no complications during device placement, and follow-up interrogations revealed no abnormalities. The patient denied other gastrointestinal symptoms or trauma to the pacer site area.
Her abdomen was soft with mild generalized tenderness, and the surgical scars from GES placement were well-healed. Device interrogation revealed settings at 2.5 volts, and impedance at 503 ohms. AP and lateral abdominal X-ray revealed proper lead placement. The decision was made to shut off the device, which ceased the shock sensations. Upper endoscopy revealed linear, subepithelial protrusions along the greater curvature of the antrum, located approximately 10.0 cm from the pylorus, consistent with typical gastric stimulator leads (Figure 1). After surgical consultation, stimulator leads were replaced, and the patient was discharged without continued symptoms.
Discussion: Adverse effects related to GES use include disruption of lead placement, erosion in the submucosa or mucosa, and obstruction, with generator site infection as the most common issue. Studies have shown that lead penetration into the gastric lumen occurs in 3% of patients, with 16% of patients requiring a corrective surgical procedure. This patient’s presentation demonstrates a rare event after GES placement. Typically, leads are placed in the muscularis propria of the greater gastric curvature. Lead displacement can be detected either on imaging or if impedance values are outside the normal range; however, neither of those occurred in this patient. Abnormal presentations demonstrated by patients with GES should be evaluated thoroughly, with suspicion for hardware malposition. Diagnostic work-up with endoscopy can guide further management.

Figure: Figure A: Upper endoscopy revealing linear, subepithelial protrusions at the greater curvature of the antrum, located approximately 10 cm from the pylorus, consistent with typical gastric stimulator leads
Figure B: Abdominal x-ray showing an anterior view of gastric pacer generator and lead placement
Figure B: Abdominal x-ray showing an anterior view of gastric pacer generator and lead placement
Disclosures:
Christo Mathew indicated no relevant financial relationships.
Peter Nguyen indicated no relevant financial relationships.
Patrick Harris indicated no relevant financial relationships.
Reena Chokshi indicated no relevant financial relationships.
Christo Mathew, MD, Peter Nguyen, MD, Patrick Harris, MD, Reena Chokshi, MD. C0727 - Elucidating with Endoscopy: Investigating Surprising Symptoms in a Patient With a Gastric Electrical Stimulator, ACG 2022 Annual Scientific Meeting Abstracts. Charlotte, NC: American College of Gastroenterology.
