Back

Poster Session C - Monday Afternoon
Category: Liver
C0607 - Bupivacaine-Induced Hepatotoxicity in a Healthy Patient Without Chronic Liver Disease After Shoulder Arthroscopy
Monday, October 24, 2022
3:00 PM – 5:00 PM ET
Location: Crown Ballroom

Has Audio

Omer Chowdhury, DO
The University of Texas Health Science Center at Tyler
Tyler, TX
Presenting Author(s)
Omer Chowdhury, DO1, Ilanko Upendran, MD2
1The University of Texas Health Science Center at Tyler, Tyler, TX; 2UT Health East Texas Physicians, Tyler, TX
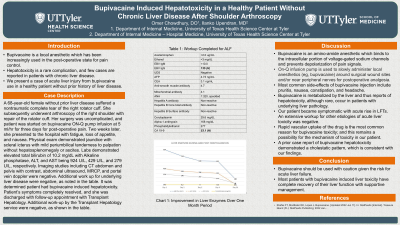
Introduction: Bupivacaine is a local anesthetic which has been increasingly used in the post-operative state for pain control. Hepatotoxicity is a rare complication, and few cases are reported in patients with chronic liver disease. We present a case of acute liver injury from bupivacaine use in a healthy patient without prior history of liver disease.
Case Description/Methods: A 68-year-old female with a past medical history of primary hypertension and recent nontraumatic complete tear of the right rotator cuff, presents to the hospital with fatigue, loss of appetite, and nausea. She recently underwent an arthroscopy of the right shoulder with repair of the rotator cuff two weeks prior. Her surgery was uncomplicated, and patient was started on bupivacaine ONQ pump infusion at 5 ml/hr for three days for post-operative pain. Further history reveals patient is non-alcoholic without prior liver disease, including cirrhosis. Review of systems is concerning for associated generalized abdominal discomfort. Physical exam demonstrated jaundice with scleral icterus with mild periumbilical tenderness to palpation without hepatosplenomegaly or ascites. Labs demonstrated elevated total bilirubin of 10.2 mg/dL with Alkaline phosphatase, ALT, and AST being 924 U/L, 429 U/L, and 279 U/L, respectively. Imaging studies including CT abdomen and pelvis with contrast, abdominal ultrasound, MRCP, and portal vein doppler were negative. Additional work up for underlying liver disease including acetaminophen and ethanol levels, SARS-CoV2, Hepatitis panel, EBV antigen, and urine toxicology were negative. It was determined patient had bupivacaine induced hepatotoxicity. Patient’s health improved with conservative management and she was discharged with instructions for close monitoring of her LFTs.
Discussion: Bupivacaine is an amino-amide anesthetic which binds to the intracellular portion of voltage-gated sodium channels and prevents depolarization of pain signals. It is metabolized by the liver and thus reports of hepatotoxicity, although rare, occur in patients with underlying liver pathology. Our patient became symptomatic with acute rise in LFTs. An extensive workup for other etiologies of acute liver toxicity was negative. Rapid vascular uptake of the drug is the most common reason for bupivacaine toxicity; and this remains a possibility for the mechanism of toxicity in our patient. A prior case report of bupivacaine hepatotoxicity demonstrated a cholestatic pattern, which is consistent with our findings.
Disclosures:
Omer Chowdhury, DO1, Ilanko Upendran, MD2. C0607 - Bupivacaine-Induced Hepatotoxicity in a Healthy Patient Without Chronic Liver Disease After Shoulder Arthroscopy, ACG 2022 Annual Scientific Meeting Abstracts. Charlotte, NC: American College of Gastroenterology.
1The University of Texas Health Science Center at Tyler, Tyler, TX; 2UT Health East Texas Physicians, Tyler, TX
Introduction: Bupivacaine is a local anesthetic which has been increasingly used in the post-operative state for pain control. Hepatotoxicity is a rare complication, and few cases are reported in patients with chronic liver disease. We present a case of acute liver injury from bupivacaine use in a healthy patient without prior history of liver disease.
Case Description/Methods: A 68-year-old female with a past medical history of primary hypertension and recent nontraumatic complete tear of the right rotator cuff, presents to the hospital with fatigue, loss of appetite, and nausea. She recently underwent an arthroscopy of the right shoulder with repair of the rotator cuff two weeks prior. Her surgery was uncomplicated, and patient was started on bupivacaine ONQ pump infusion at 5 ml/hr for three days for post-operative pain. Further history reveals patient is non-alcoholic without prior liver disease, including cirrhosis. Review of systems is concerning for associated generalized abdominal discomfort. Physical exam demonstrated jaundice with scleral icterus with mild periumbilical tenderness to palpation without hepatosplenomegaly or ascites. Labs demonstrated elevated total bilirubin of 10.2 mg/dL with Alkaline phosphatase, ALT, and AST being 924 U/L, 429 U/L, and 279 U/L, respectively. Imaging studies including CT abdomen and pelvis with contrast, abdominal ultrasound, MRCP, and portal vein doppler were negative. Additional work up for underlying liver disease including acetaminophen and ethanol levels, SARS-CoV2, Hepatitis panel, EBV antigen, and urine toxicology were negative. It was determined patient had bupivacaine induced hepatotoxicity. Patient’s health improved with conservative management and she was discharged with instructions for close monitoring of her LFTs.
Discussion: Bupivacaine is an amino-amide anesthetic which binds to the intracellular portion of voltage-gated sodium channels and prevents depolarization of pain signals. It is metabolized by the liver and thus reports of hepatotoxicity, although rare, occur in patients with underlying liver pathology. Our patient became symptomatic with acute rise in LFTs. An extensive workup for other etiologies of acute liver toxicity was negative. Rapid vascular uptake of the drug is the most common reason for bupivacaine toxicity; and this remains a possibility for the mechanism of toxicity in our patient. A prior case report of bupivacaine hepatotoxicity demonstrated a cholestatic pattern, which is consistent with our findings.
Disclosures:
Omer Chowdhury indicated no relevant financial relationships.
Ilanko Upendran indicated no relevant financial relationships.
Omer Chowdhury, DO1, Ilanko Upendran, MD2. C0607 - Bupivacaine-Induced Hepatotoxicity in a Healthy Patient Without Chronic Liver Disease After Shoulder Arthroscopy, ACG 2022 Annual Scientific Meeting Abstracts. Charlotte, NC: American College of Gastroenterology.
