Back
Poster Session A - Sunday Afternoon
Category: Liver
A0568 - A Bactrim-Induced Liver Failure Requiring a Transplant: A Report of a Rare Side Effect of a Commonly Used Drug!
Sunday, October 23, 2022
5:00 PM – 7:00 PM ET
Location: Crown Ballroom
- RA
Rawan Aljaras, MD
Indiana University School of Medicine
Indianapolis, Indiana
Presenting Author(s)
Rawan Aljaras, MD1, Maryam Haider, MD2, Ahmad Karkash, MD3, Bianca Nicole Puello Yocum, MD3, Razan Aljaras, MD3, Gerardo Calderon, MD1
1Indiana University, Indianapolis, IN; 2Wayne State University/Detroit Medical Center Sinai Grace Hospital, Detroit, MI; 3Indiana University School of Medicine, Indianapolis, IN
Introduction: Drug-induced Liver Injury (DILI) is an important cause of acute liver failure cases in the United States. Multiple drugs including prescription, over-the-counter, and herbal products can cause hepatotoxicity through a variety of mechanisms. Clinical suspicion is key in diagnosing DILI. Sulfamethoxazole with trimethoprim (Bactrim or TMP/SMX) is a fixed antibiotic combination that is very commonly used in clinical practice, and as many others, this combination has been linked to rare instances of acute liver failure, sometimes even requiring a liver transplant as represented by this case.
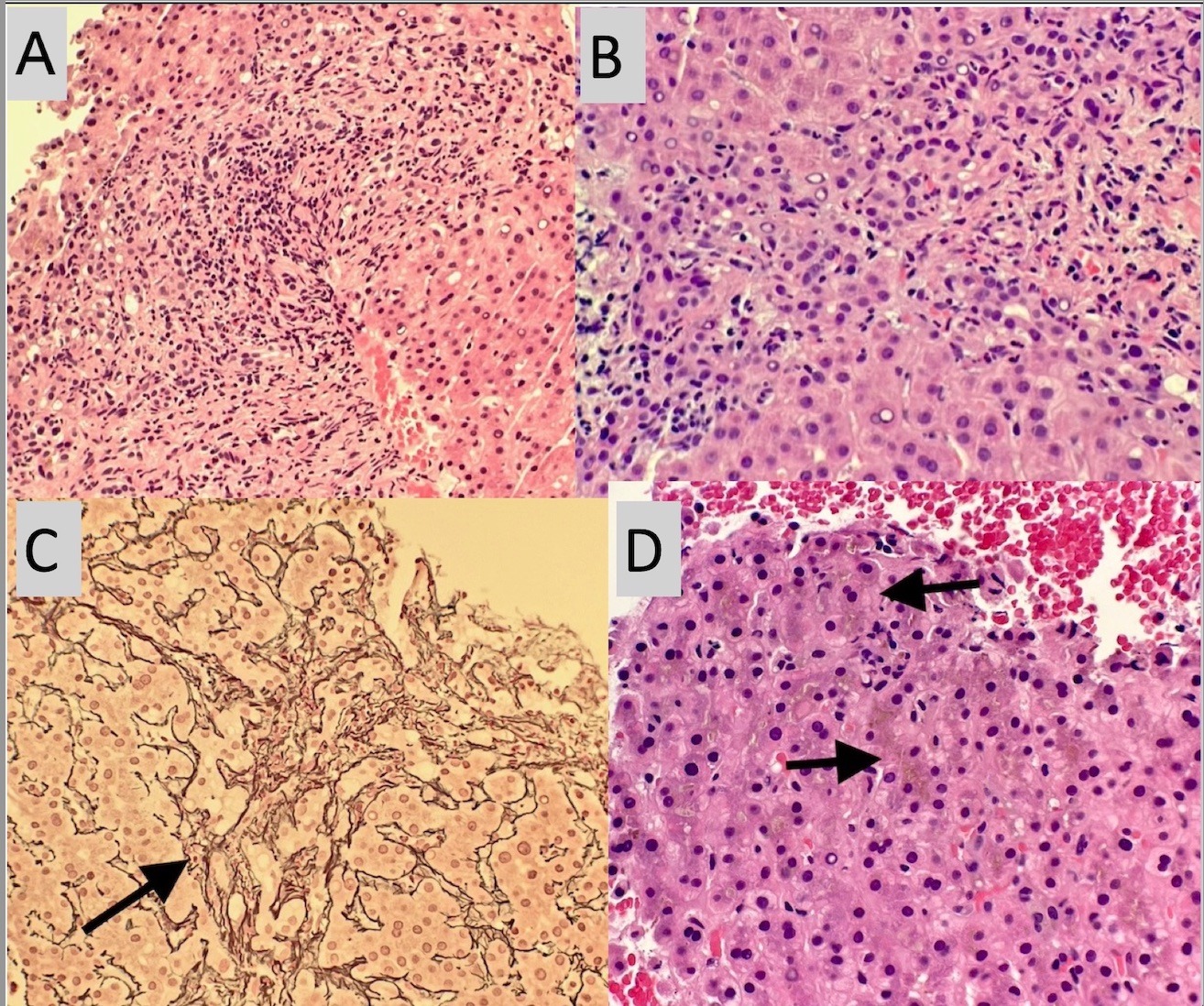
Case Description/Methods: We present a case of a 38 year old female patient who presented with jaundice a month after she completed a 5 day course of Bactrim for a urinary tract infection. Initial labs were notable for cholestatic pattern of liver injury. Extensive infectious and autoimmune workup came back negative. MRCP and ERCP were both done and noted unremarkable with no biliary tract abnormalities. Liver biopsy with cholestasis and liver injury pattern described in (Figures A,B,C,&D). Patient was started on oral ursodiol and steroids, with continued trend up in liver enzymes and eventually the development of ascites. Patient had a repeat liver biopsy few months after which showed cirrhosis and cholestasis. Roughly, 18 months after exposure to Bactrim, patient got a liver transplant.
Discussion: As fascinated as the patient could be, a simple urinary tract infection ended up with her needing an organ transplant and fortunately she was able get one. Bactrim is known for its potential to cause idiosyncratic liver injury that has features of drug-allergy or hypersensitivity. Three forms of Bactrim induced liver damage have been described; hepatocellular, mixed hepatocellular cholestatic, and (more recently) bile duct injury with ductopenia or Vanishing Bile Duct Syndrome. The onset of symptoms usually occurs within a few days of ingestion but can take up to 1–2 months. Diagnosis is suspected from the clinical presentation, and absence of other causes, in addition to suggestive changes on liver biopsy. Treatment is generally supportive; liver transplantation has been successful for both fulminant hepatic failure and vanishing bile duct syndrome of varying causes.
Disclosures:
Rawan Aljaras, MD1, Maryam Haider, MD2, Ahmad Karkash, MD3, Bianca Nicole Puello Yocum, MD3, Razan Aljaras, MD3, Gerardo Calderon, MD1. A0568 - A Bactrim-Induced Liver Failure Requiring a Transplant: A Report of a Rare Side Effect of a Commonly Used Drug!, ACG 2022 Annual Scientific Meeting Abstracts. Charlotte, NC: American College of Gastroenterology.
1Indiana University, Indianapolis, IN; 2Wayne State University/Detroit Medical Center Sinai Grace Hospital, Detroit, MI; 3Indiana University School of Medicine, Indianapolis, IN
Introduction: Drug-induced Liver Injury (DILI) is an important cause of acute liver failure cases in the United States. Multiple drugs including prescription, over-the-counter, and herbal products can cause hepatotoxicity through a variety of mechanisms. Clinical suspicion is key in diagnosing DILI. Sulfamethoxazole with trimethoprim (Bactrim or TMP/SMX) is a fixed antibiotic combination that is very commonly used in clinical practice, and as many others, this combination has been linked to rare instances of acute liver failure, sometimes even requiring a liver transplant as represented by this case.
Case Description/Methods: We present a case of a 38 year old female patient who presented with jaundice a month after she completed a 5 day course of Bactrim for a urinary tract infection. Initial labs were notable for cholestatic pattern of liver injury. Extensive infectious and autoimmune workup came back negative. MRCP and ERCP were both done and noted unremarkable with no biliary tract abnormalities. Liver biopsy with cholestasis and liver injury pattern described in (Figures A,B,C,&D). Patient was started on oral ursodiol and steroids, with continued trend up in liver enzymes and eventually the development of ascites. Patient had a repeat liver biopsy few months after which showed cirrhosis and cholestasis. Roughly, 18 months after exposure to Bactrim, patient got a liver transplant.
Discussion: As fascinated as the patient could be, a simple urinary tract infection ended up with her needing an organ transplant and fortunately she was able get one. Bactrim is known for its potential to cause idiosyncratic liver injury that has features of drug-allergy or hypersensitivity. Three forms of Bactrim induced liver damage have been described; hepatocellular, mixed hepatocellular cholestatic, and (more recently) bile duct injury with ductopenia or Vanishing Bile Duct Syndrome. The onset of symptoms usually occurs within a few days of ingestion but can take up to 1–2 months. Diagnosis is suspected from the clinical presentation, and absence of other causes, in addition to suggestive changes on liver biopsy. Treatment is generally supportive; liver transplantation has been successful for both fulminant hepatic failure and vanishing bile duct syndrome of varying causes.

Figure: A- H&E stained liver biopsy demonstrates moderate portal inflammatory infiltrate consisting of neutrophils, lymphocytes, and eosinophils. There is a marked ductular reaction.
B- H&E stained liver biopsy demonstrates moderate portal inflammatory infiltrate consisting of neutrophils, lymphocytes, and eosinophils. There is a marked ductulaR reaction.
C- Reticulin stain demonstrates mild portal fibrosis (arrow) with preserved hepatic architecture.
D- H&E stained liver biopsy demonstrating marked cholestasis (arrows).
B- H&E stained liver biopsy demonstrates moderate portal inflammatory infiltrate consisting of neutrophils, lymphocytes, and eosinophils. There is a marked ductulaR reaction.
C- Reticulin stain demonstrates mild portal fibrosis (arrow) with preserved hepatic architecture.
D- H&E stained liver biopsy demonstrating marked cholestasis (arrows).
Disclosures:
Rawan Aljaras indicated no relevant financial relationships.
Maryam Haider indicated no relevant financial relationships.
Ahmad Karkash indicated no relevant financial relationships.
Bianca Nicole Puello Yocum indicated no relevant financial relationships.
Razan Aljaras indicated no relevant financial relationships.
Gerardo Calderon indicated no relevant financial relationships.
Rawan Aljaras, MD1, Maryam Haider, MD2, Ahmad Karkash, MD3, Bianca Nicole Puello Yocum, MD3, Razan Aljaras, MD3, Gerardo Calderon, MD1. A0568 - A Bactrim-Induced Liver Failure Requiring a Transplant: A Report of a Rare Side Effect of a Commonly Used Drug!, ACG 2022 Annual Scientific Meeting Abstracts. Charlotte, NC: American College of Gastroenterology.
