Back


Poster Session D - Tuesday Morning
Category: Liver
D0504 - Prevalence of Hepatitis B Virus (HBV) and Latent Tuberculosis Co-Infection and Risk of Drug-Induced Liver Injury Across Two Large HBV Cohorts in the United States
Tuesday, October 25, 2022
10:00 AM – 12:00 PM ET
Location: Crown Ballroom

Has Audio

Robert Wong, MD, MD, FACG
Stanford University School of Medicine/Veterans Affairs Palo Alto Healthcare System
Palo Alto, CA
Presenting Author(s)
Robert Wong, MD, MD, FACG1, Loralee B. Rupp, MS, MBA2, Mei Lu, PhD2, Zeyuan Yang, MPH3, Yihe G. Daida, PhD4, Mark A. Schmidt, PhD, MPH5, Joseph A. Boscarino, PhD, MPH6, Stuart Gordon, MD2, Amit Chitnis, MD, MPH7
1Stanford University School of Medicine/Veterans Affairs Palo Alto Healthcare System, Palo Alto, CA; 2Henry Ford Health System, Detroit, MI; 3Veterans Affairs Palo Alto Healthcare System, Palo Alto, CA; 4Kaiser Permanente Hawaii, Honolulu, HI; 5Kaiser Permanente Center for Health Research, Portland, OR; 6Geisinger Clinic, Mahwah, NJ; 7Alameda County Public Health Department, San Leandro, CA
Introduction: Screening for hepatitis B virus (HBV) infection prior to starting tuberculosis (TB) treatment is important because HBV-TB co-infected patients have increased risk of drug-induced liver injury (DILI). However, there are limited real-world data on epidemiology of HBV-latent TB infection (LTBI) in the US. We evaluated prevalence and predictors of HBV-LTBI co-infection and DILI risk among two distinct US chronic HBV cohorts.
Methods: Adults with chronic HBV from 2010–2020 were identified among the Chronic Hepatitis Cohort Study (CHeCS) and the Veterans Affairs national chronic HBV cohort (VA-HBV). HBV-LTBI co-infection was identified based on laboratory data (TB-Quantiferon), ICD-9/10 codes, and prescription data. DILI was identified based on established definitions that incorporated ICD-9/10 codes and changes in alanine aminotransferase levels following start of LTBI treatment.
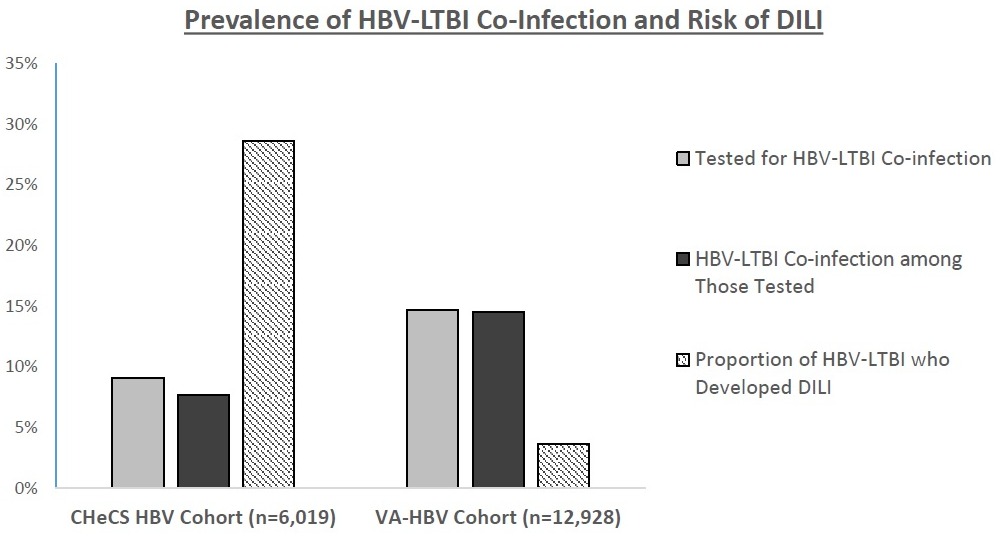
Results: Among 6,019 chronic HBV patients in the CHeCS cohort (44% female; 47% age 18-39y, 39% age 40-59y, 14% age >60y; 47% Asian, 20% non-Hispanic white (NHW), 14% African American (AA), 1% Hispanic; 3% HCV; 6% HIV), 9.1% were tested for TB, among which 7.7% had HBV-LTBI. Significantly higher odds of HBV-LTBI were observed in women vs. men (13% vs. 5%, OR 2.57, 95% CI 1.36-4.85 p< 0.01), but no other significant differences were observed. Among HBV-LTBI patients that received LTBI treatment, 28.6% developed DILI. Among 12,928 predominantly U.S.-born chronic HBV patients in the VA-HBV cohort (94% male; 6% age 18-39y, 30% age 40-59y, 65% age >60y; 10% Asian, 42% AA, 39% NHW, 2% Hispanic; 86% US-born; 15% HCV; 2.3% HIV), 14.7% were tested for TB, among which 14.5% had HBV-LTBI. Significantly higher odds of HBV-LTBI were observed in AA vs. NHW (15% vs. 12%, OR 1.70, 95% CI 1.18-2.43, p< 0.01) and in non-US born vs. US-born (25% vs. 13%, OR 2.13, 95% CI 1.34-4.00, p< 0.05). Among HBV-LTBI patients that received LTBI treatment, the proportion of patients that developed DILI was 3.6%.
Discussion: Among two large distinct US cohorts of chronic HBV patients, testing for LTBI was infrequent despite relatively high prevalence of HBV-LTBI. While nearly 30% of HBV-LTBI patients in the predominantly Asian and younger CHeCS cohort developed DILI, only 3.6% in the predominantly older, US born VA-HBV cohort developed DILI. Better understanding risk factors for DILI among HBV-LTBI patients can help guide clinicians to appropriately modify LTBI treatment to reduce DILI risk. Supported by ACG Clinical Research Award.
Disclosures:
Robert Wong, MD, MD, FACG1, Loralee B. Rupp, MS, MBA2, Mei Lu, PhD2, Zeyuan Yang, MPH3, Yihe G. Daida, PhD4, Mark A. Schmidt, PhD, MPH5, Joseph A. Boscarino, PhD, MPH6, Stuart Gordon, MD2, Amit Chitnis, MD, MPH7. D0504 - Prevalence of Hepatitis B Virus (HBV) and Latent Tuberculosis Co-Infection and Risk of Drug-Induced Liver Injury Across Two Large HBV Cohorts in the United States, ACG 2022 Annual Scientific Meeting Abstracts. Charlotte, NC: American College of Gastroenterology.
1Stanford University School of Medicine/Veterans Affairs Palo Alto Healthcare System, Palo Alto, CA; 2Henry Ford Health System, Detroit, MI; 3Veterans Affairs Palo Alto Healthcare System, Palo Alto, CA; 4Kaiser Permanente Hawaii, Honolulu, HI; 5Kaiser Permanente Center for Health Research, Portland, OR; 6Geisinger Clinic, Mahwah, NJ; 7Alameda County Public Health Department, San Leandro, CA
Introduction: Screening for hepatitis B virus (HBV) infection prior to starting tuberculosis (TB) treatment is important because HBV-TB co-infected patients have increased risk of drug-induced liver injury (DILI). However, there are limited real-world data on epidemiology of HBV-latent TB infection (LTBI) in the US. We evaluated prevalence and predictors of HBV-LTBI co-infection and DILI risk among two distinct US chronic HBV cohorts.
Methods: Adults with chronic HBV from 2010–2020 were identified among the Chronic Hepatitis Cohort Study (CHeCS) and the Veterans Affairs national chronic HBV cohort (VA-HBV). HBV-LTBI co-infection was identified based on laboratory data (TB-Quantiferon), ICD-9/10 codes, and prescription data. DILI was identified based on established definitions that incorporated ICD-9/10 codes and changes in alanine aminotransferase levels following start of LTBI treatment.
Results: Among 6,019 chronic HBV patients in the CHeCS cohort (44% female; 47% age 18-39y, 39% age 40-59y, 14% age >60y; 47% Asian, 20% non-Hispanic white (NHW), 14% African American (AA), 1% Hispanic; 3% HCV; 6% HIV), 9.1% were tested for TB, among which 7.7% had HBV-LTBI. Significantly higher odds of HBV-LTBI were observed in women vs. men (13% vs. 5%, OR 2.57, 95% CI 1.36-4.85 p< 0.01), but no other significant differences were observed. Among HBV-LTBI patients that received LTBI treatment, 28.6% developed DILI. Among 12,928 predominantly U.S.-born chronic HBV patients in the VA-HBV cohort (94% male; 6% age 18-39y, 30% age 40-59y, 65% age >60y; 10% Asian, 42% AA, 39% NHW, 2% Hispanic; 86% US-born; 15% HCV; 2.3% HIV), 14.7% were tested for TB, among which 14.5% had HBV-LTBI. Significantly higher odds of HBV-LTBI were observed in AA vs. NHW (15% vs. 12%, OR 1.70, 95% CI 1.18-2.43, p< 0.01) and in non-US born vs. US-born (25% vs. 13%, OR 2.13, 95% CI 1.34-4.00, p< 0.05). Among HBV-LTBI patients that received LTBI treatment, the proportion of patients that developed DILI was 3.6%.
Discussion: Among two large distinct US cohorts of chronic HBV patients, testing for LTBI was infrequent despite relatively high prevalence of HBV-LTBI. While nearly 30% of HBV-LTBI patients in the predominantly Asian and younger CHeCS cohort developed DILI, only 3.6% in the predominantly older, US born VA-HBV cohort developed DILI. Better understanding risk factors for DILI among HBV-LTBI patients can help guide clinicians to appropriately modify LTBI treatment to reduce DILI risk. Supported by ACG Clinical Research Award.

Figure: Prevalence of HBV-LTBI Co-Infection and Risk of DILI
Disclosures:
Robert Wong: Gilead Sciences – Consultant, Grant/Research Support.
Loralee Rupp: AbbVie – Grant/Research Support. Gilead Sciences – Grant/Research Support. GlaxoSmithKline – Grant/Research Support. Intercept Pharmaceuticals – Grant/Research Support.
Mei Lu indicated no relevant financial relationships.
Zeyuan Yang indicated no relevant financial relationships.
Yihe Daida: GSK – Grant/Research Support. Vir Biotechnology – Grant/Research Support.
Mark Schmidt indicated no relevant financial relationships.
Joseph Boscarino indicated no relevant financial relationships.
Stuart Gordon indicated no relevant financial relationships.
Amit Chitnis indicated no relevant financial relationships.
Robert Wong, MD, MD, FACG1, Loralee B. Rupp, MS, MBA2, Mei Lu, PhD2, Zeyuan Yang, MPH3, Yihe G. Daida, PhD4, Mark A. Schmidt, PhD, MPH5, Joseph A. Boscarino, PhD, MPH6, Stuart Gordon, MD2, Amit Chitnis, MD, MPH7. D0504 - Prevalence of Hepatitis B Virus (HBV) and Latent Tuberculosis Co-Infection and Risk of Drug-Induced Liver Injury Across Two Large HBV Cohorts in the United States, ACG 2022 Annual Scientific Meeting Abstracts. Charlotte, NC: American College of Gastroenterology.
