Back

Poster Session D - Tuesday Morning
Category: Liver
D0519 - Safety and Efficacy of Oral TLR8 Agonist, Selgantolimod, in Viremic Adult Patients With Chronic Hepatitis B
Tuesday, October 25, 2022
10:00 AM – 12:00 PM ET
Location: Crown Ballroom

Has Audio
- HJ
Harry Janssen, MD, PhD
Toronto Centre for Liver Disease, Toronto General Hospital, University Health Network
Toronto, ON, Canada
Presenting Author(s)
Award: Presidential Poster Award
Harry Janssen, MD, PhD1, Young-Suk Lim, MD2, Hyung Joon Kim, MD3, Cheng-Hao Tseng, MD4, Carla Coffin, MD5, Magdy Elkashab, MD1, Sang Hoon Ahn, MD6, Anh-Hoa Nguyen, PhD7, Diana Chen, PhD7, Jeffrey Wallin, PhD7, Susana Tan, MD7, Jenny Yang, 7, Anuj Gaggar, MD7, Diana Brainard, MD7, Scott Fung, MD1, Yoon Jun Kim, PhD8, Jia-Horng Kao, MD9, Wan-Long Chuang, MD10, Anna Brooks, PhD11, Rod Dunbar, MD, PhD11
1Toronto Centre for Liver Disease, Toronto General Hospital, University Health Network, Toronto, ON, Canada; 2University of Ulsan College of Medicine, Songpa-gu, Seoul-t'ukpyolsi, Republic of Korea; 3Chung-Ang University, School of Medicine, Dongjak-gu, Seoul-t'ukpyolsi, Republic of Korea; 4E-Da Hospital, Kaohsiung City, Kaohsiung, Taiwan; 5University of Calgary, Calgary, AB, Canada; 6Severance Hospital, Sinchon-dong, Seoul-t'ukpyolsi, Republic of Korea; 7Gilead Sciences, Inc., Foster City, CA; 8Soul National University College of Medicine, Jongno-gu, Seoul-t'ukpyolsi, Republic of Korea; 9National Taiwan University Hospital, Taipei City, Taipei, Taiwan; 10Kaohsiung Medical University Hospital, Kaohsiung City, Kaohsiung, Taiwan; 11University of Auckland, Auckland Central, Auckland, New Zealand
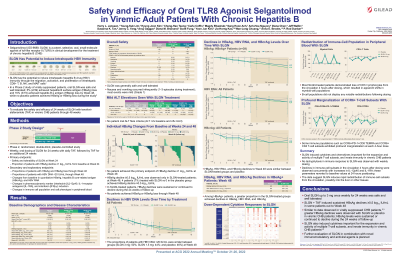
Introduction: Selgantolimod (GS-9688, SLGN) is an oral, Toll-like receptor 8 agonist in clinical development for the treatment of chronic hepatitis B (CHB). Here we present the results through week 48 on the safety and efficacy of 24 weeks of SLGN treatment in viremic CHB patients.
Methods: In this multicenter, double-blind, phase 2 study, viremic CHB patients were randomized (2:2:1) to SLGN 3 mg, 1.5 mg, and placebo (PBO) once a week for 24 weeks in combination with tenofovir alafenamide. Safety assessments included monitoring of treatment emergent adverse events (TEAE) and laboratory abnor-malities. The primary efficacy end point was the proportion of patients with ≥1 log10IU/ml decline in HBsAg levels from baseline at week 24. Exploratory end points include changes in pharmacodynamic (PD) markers (e.g. IL-12p40 and IL-1RA) and changes in peripheral T- cell, myeloid and NK-cell subsets.
Results: 67 patients (39 HBeAg-positive) were randomized. Baseline characteristics were similar across groups: majority were Asian (98.5%), male (58%) with a median (IQR) age of 47 (35–54) years, HBsAg level of 4.1 (3.5–4.7) log10IU/ml, and HBV DNA level of 7.5 (5.4–8.3) log10IU/ml. No patients achieved the primary end point of ≥1 log10IU/ml decline in HBsAg levels at week 24; however, 3 (6%) patients in the SLGN-treated achieved HBsAg decline ≥0.5 log10IU/ml compared to none in the placebo group. At week 48, 4 (7.4%) patients in the SLGN-treated group (including the 3 subjects at week 24) while none in the placebo group achieved HBsAg decline ≥0.5 log10IU/ml. Most frequent (≥10% SLGN-treated) TEAE (SLGN v PBO) were: nausea (26% v 0%), headache (15% v 15%), vomiting (17% v 0%), fatigue (15% v 0), and dizziness (11% v 0%). Grade ≥3 TEAE were observed in 0 (SLGN) v 7.7% (PBO) subjects; 1 subject (SLGN 3 mg) discontinued study due to TEAE of vomiting and abdominal pain. Most patients treated with SLGN showed decline of immune cell subsets in the circulation 4 h after dosing, concurrent with increases of circulating IL-12p40 and IL-1RA. Cell populations that decreased in the circulation included effector and memory T cell subsets. These parameters reverted to baseline values at 24 h post-dosing.
Discussion: Oral SLGN up to 3 mg once weekly for 24 weeks is safe and well-tolerated. SLGN can induce sustained HBsAg declines of ≥0.5 log10IU/ml in some patients out to Week 48. Further evaluation of SLGN in combination with immunomodulatory and antiviral agents is planned.

Disclosures:
Harry Janssen, MD, PhD1, Young-Suk Lim, MD2, Hyung Joon Kim, MD3, Cheng-Hao Tseng, MD4, Carla Coffin, MD5, Magdy Elkashab, MD1, Sang Hoon Ahn, MD6, Anh-Hoa Nguyen, PhD7, Diana Chen, PhD7, Jeffrey Wallin, PhD7, Susana Tan, MD7, Jenny Yang, 7, Anuj Gaggar, MD7, Diana Brainard, MD7, Scott Fung, MD1, Yoon Jun Kim, PhD8, Jia-Horng Kao, MD9, Wan-Long Chuang, MD10, Anna Brooks, PhD11, Rod Dunbar, MD, PhD11. D0519 - Safety and Efficacy of Oral TLR8 Agonist, Selgantolimod, in Viremic Adult Patients With Chronic Hepatitis B, ACG 2022 Annual Scientific Meeting Abstracts. Charlotte, NC: American College of Gastroenterology.
Harry Janssen, MD, PhD1, Young-Suk Lim, MD2, Hyung Joon Kim, MD3, Cheng-Hao Tseng, MD4, Carla Coffin, MD5, Magdy Elkashab, MD1, Sang Hoon Ahn, MD6, Anh-Hoa Nguyen, PhD7, Diana Chen, PhD7, Jeffrey Wallin, PhD7, Susana Tan, MD7, Jenny Yang, 7, Anuj Gaggar, MD7, Diana Brainard, MD7, Scott Fung, MD1, Yoon Jun Kim, PhD8, Jia-Horng Kao, MD9, Wan-Long Chuang, MD10, Anna Brooks, PhD11, Rod Dunbar, MD, PhD11
1Toronto Centre for Liver Disease, Toronto General Hospital, University Health Network, Toronto, ON, Canada; 2University of Ulsan College of Medicine, Songpa-gu, Seoul-t'ukpyolsi, Republic of Korea; 3Chung-Ang University, School of Medicine, Dongjak-gu, Seoul-t'ukpyolsi, Republic of Korea; 4E-Da Hospital, Kaohsiung City, Kaohsiung, Taiwan; 5University of Calgary, Calgary, AB, Canada; 6Severance Hospital, Sinchon-dong, Seoul-t'ukpyolsi, Republic of Korea; 7Gilead Sciences, Inc., Foster City, CA; 8Soul National University College of Medicine, Jongno-gu, Seoul-t'ukpyolsi, Republic of Korea; 9National Taiwan University Hospital, Taipei City, Taipei, Taiwan; 10Kaohsiung Medical University Hospital, Kaohsiung City, Kaohsiung, Taiwan; 11University of Auckland, Auckland Central, Auckland, New Zealand
Introduction: Selgantolimod (GS-9688, SLGN) is an oral, Toll-like receptor 8 agonist in clinical development for the treatment of chronic hepatitis B (CHB). Here we present the results through week 48 on the safety and efficacy of 24 weeks of SLGN treatment in viremic CHB patients.
Methods: In this multicenter, double-blind, phase 2 study, viremic CHB patients were randomized (2:2:1) to SLGN 3 mg, 1.5 mg, and placebo (PBO) once a week for 24 weeks in combination with tenofovir alafenamide. Safety assessments included monitoring of treatment emergent adverse events (TEAE) and laboratory abnor-malities. The primary efficacy end point was the proportion of patients with ≥1 log10IU/ml decline in HBsAg levels from baseline at week 24. Exploratory end points include changes in pharmacodynamic (PD) markers (e.g. IL-12p40 and IL-1RA) and changes in peripheral T- cell, myeloid and NK-cell subsets.
Results: 67 patients (39 HBeAg-positive) were randomized. Baseline characteristics were similar across groups: majority were Asian (98.5%), male (58%) with a median (IQR) age of 47 (35–54) years, HBsAg level of 4.1 (3.5–4.7) log10IU/ml, and HBV DNA level of 7.5 (5.4–8.3) log10IU/ml. No patients achieved the primary end point of ≥1 log10IU/ml decline in HBsAg levels at week 24; however, 3 (6%) patients in the SLGN-treated achieved HBsAg decline ≥0.5 log10IU/ml compared to none in the placebo group. At week 48, 4 (7.4%) patients in the SLGN-treated group (including the 3 subjects at week 24) while none in the placebo group achieved HBsAg decline ≥0.5 log10IU/ml. Most frequent (≥10% SLGN-treated) TEAE (SLGN v PBO) were: nausea (26% v 0%), headache (15% v 15%), vomiting (17% v 0%), fatigue (15% v 0), and dizziness (11% v 0%). Grade ≥3 TEAE were observed in 0 (SLGN) v 7.7% (PBO) subjects; 1 subject (SLGN 3 mg) discontinued study due to TEAE of vomiting and abdominal pain. Most patients treated with SLGN showed decline of immune cell subsets in the circulation 4 h after dosing, concurrent with increases of circulating IL-12p40 and IL-1RA. Cell populations that decreased in the circulation included effector and memory T cell subsets. These parameters reverted to baseline values at 24 h post-dosing.
Discussion: Oral SLGN up to 3 mg once weekly for 24 weeks is safe and well-tolerated. SLGN can induce sustained HBsAg declines of ≥0.5 log10IU/ml in some patients out to Week 48. Further evaluation of SLGN in combination with immunomodulatory and antiviral agents is planned.
Disclosures:
Harry Janssen indicated no relevant financial relationships.
Young-Suk Lim indicated no relevant financial relationships.
Hyung Joon Kim indicated no relevant financial relationships.
Cheng-Hao Tseng indicated no relevant financial relationships.
Carla Coffin indicated no relevant financial relationships.
Magdy Elkashab indicated no relevant financial relationships.
Sang Hoon Ahn indicated no relevant financial relationships.
Anh-Hoa Nguyen: Gilead Sciences, Inc. – Employee.
Diana Chen: Gilead Sciences, Inc. – Employee.
Jeffrey Wallin: Gilead Sciences, Inc. – Employee.
Susana Tan: Gilead Sciences, Inc. – Employee.
Jenny Yang: Gilead Sciences, Inc. – Employee.
Anuj Gaggar: Gilead Sciences, Inc. – Employee.
Diana Brainard: Gilead Sciences, Inc. – Employee.
Scott Fung: AbbVie – Speakers Bureau. Assembly Biosciences – Grant/Research Support. Gilead Sciences, Inc. – Grant/Research Support, Speakers Bureau. Janssen – Grant/Research Support.
Yoon Jun Kim indicated no relevant financial relationships.
Jia-Horng Kao indicated no relevant financial relationships.
Wan-Long Chuang indicated no relevant financial relationships.
Anna Brooks indicated no relevant financial relationships.
Rod Dunbar indicated no relevant financial relationships.
Harry Janssen, MD, PhD1, Young-Suk Lim, MD2, Hyung Joon Kim, MD3, Cheng-Hao Tseng, MD4, Carla Coffin, MD5, Magdy Elkashab, MD1, Sang Hoon Ahn, MD6, Anh-Hoa Nguyen, PhD7, Diana Chen, PhD7, Jeffrey Wallin, PhD7, Susana Tan, MD7, Jenny Yang, 7, Anuj Gaggar, MD7, Diana Brainard, MD7, Scott Fung, MD1, Yoon Jun Kim, PhD8, Jia-Horng Kao, MD9, Wan-Long Chuang, MD10, Anna Brooks, PhD11, Rod Dunbar, MD, PhD11. D0519 - Safety and Efficacy of Oral TLR8 Agonist, Selgantolimod, in Viremic Adult Patients With Chronic Hepatitis B, ACG 2022 Annual Scientific Meeting Abstracts. Charlotte, NC: American College of Gastroenterology.

