Back

Poster Session D - Tuesday Morning
Category: Liver
D0525 - Autoimmune Hepatitis, SLE, and Leukocytoclastic Vasculitis Following the Moderna COVID Vaccine
Tuesday, October 25, 2022
10:00 AM – 12:00 PM ET
Location: Crown Ballroom

Has Audio

Daniel Weinblatt, DO
Cooper University Hospital
Camden, NJ
Presenting Author(s)
Daniel Weinblatt, DO, Upasana Joneja, MD, Adib Chaaya, MD
Cooper University Hospital, Camden, NJ
Introduction: There have been a small number of case reports of patients who developed autoimmune hepatitis following vaccination against COVID. (1) The following case report details the development of multiple autoimmune conditions, including AIH, SLE, and leukocytoclastic vasculitis in a patient shortly after she received the Moderna vaccine against COVID.
Case Description/Methods: A 59-year-old woman with a history of morbid obesity, CAD, HTN, HLD, and prior cholecystectomy presented to the emergency department complaining of petechial rash and fatigue that developed 10 days after receiving the Moderna COVID vaccine. Physical exam was notable only for a petechial rash involving both legs. A biopsy of the rash revealed LCV. She was found to have abnormal liver enzymes with ALT 259 U/L, AST 382 U/L, TBili 1.9 mg/dL, and Alk Phos 224 U/L. Further testing revealed an ANA with 1:1280 titer and elevated IgG level at 2,815 mg/dL. RUQ ultrasound revealed no evidence of biliary obstruction and mild CBD dilation due to cholecystectomy. Viral hepatitis, CMV, celiac panel, ceruloplasmin, ferritin, AMA, ASMA, and alpha-1 antitrypsin testing were all negative. Both her rash and liver injury improved with steroid administration but worsened with tapering. She then underwent liver biopsy, which revealed lymphoplasmacytic infiltrate and interface hepatitis that were compatible with autoimmune hepatitis. She was also found to have low complement and positive anti-dsDNA, and was diagnosed with SLE. Her liver injury improved with IV steroids, and she was then transitioned to azathioprine and tapered off of steroids without further flares.
Discussion: The diagnosis of autoimmune hepatitis was not able to be confirmed until the liver biopsy. Our patient received 7 points for the IAHG AIH diagnostic criteria, indicating definitive AIH. This case report adds to this small but growing series of patients who developed autoimmune hepatitis after receiving a COVID-19 vaccine. Because of vaccine hesitancy, providers must be able to have an informed discussion with patients about the benefits and risks. This case highlights the need for greater awareness on the part of clinicians of this rare complication.
Reference
1) Bril F. Autoimmune hepatitis developing after coronavirus disease 2019 (COVID-19) vaccine: One or even several swallows do not make a summer [published online ahead of print, 2021 Aug 10]. J Hepatol. 2021;S0168-8278(21)01965-6. doi:10.1016/j.jhep.2021.08.001
Disclosures:
Daniel Weinblatt, DO, Upasana Joneja, MD, Adib Chaaya, MD. D0525 - Autoimmune Hepatitis, SLE, and Leukocytoclastic Vasculitis Following the Moderna COVID Vaccine, ACG 2022 Annual Scientific Meeting Abstracts. Charlotte, NC: American College of Gastroenterology.
Cooper University Hospital, Camden, NJ
Introduction: There have been a small number of case reports of patients who developed autoimmune hepatitis following vaccination against COVID. (1) The following case report details the development of multiple autoimmune conditions, including AIH, SLE, and leukocytoclastic vasculitis in a patient shortly after she received the Moderna vaccine against COVID.
Case Description/Methods: A 59-year-old woman with a history of morbid obesity, CAD, HTN, HLD, and prior cholecystectomy presented to the emergency department complaining of petechial rash and fatigue that developed 10 days after receiving the Moderna COVID vaccine. Physical exam was notable only for a petechial rash involving both legs. A biopsy of the rash revealed LCV. She was found to have abnormal liver enzymes with ALT 259 U/L, AST 382 U/L, TBili 1.9 mg/dL, and Alk Phos 224 U/L. Further testing revealed an ANA with 1:1280 titer and elevated IgG level at 2,815 mg/dL. RUQ ultrasound revealed no evidence of biliary obstruction and mild CBD dilation due to cholecystectomy. Viral hepatitis, CMV, celiac panel, ceruloplasmin, ferritin, AMA, ASMA, and alpha-1 antitrypsin testing were all negative. Both her rash and liver injury improved with steroid administration but worsened with tapering. She then underwent liver biopsy, which revealed lymphoplasmacytic infiltrate and interface hepatitis that were compatible with autoimmune hepatitis. She was also found to have low complement and positive anti-dsDNA, and was diagnosed with SLE. Her liver injury improved with IV steroids, and she was then transitioned to azathioprine and tapered off of steroids without further flares.
Discussion: The diagnosis of autoimmune hepatitis was not able to be confirmed until the liver biopsy. Our patient received 7 points for the IAHG AIH diagnostic criteria, indicating definitive AIH. This case report adds to this small but growing series of patients who developed autoimmune hepatitis after receiving a COVID-19 vaccine. Because of vaccine hesitancy, providers must be able to have an informed discussion with patients about the benefits and risks. This case highlights the need for greater awareness on the part of clinicians of this rare complication.
Reference
1) Bril F. Autoimmune hepatitis developing after coronavirus disease 2019 (COVID-19) vaccine: One or even several swallows do not make a summer [published online ahead of print, 2021 Aug 10]. J Hepatol. 2021;S0168-8278(21)01965-6. doi:10.1016/j.jhep.2021.08.001

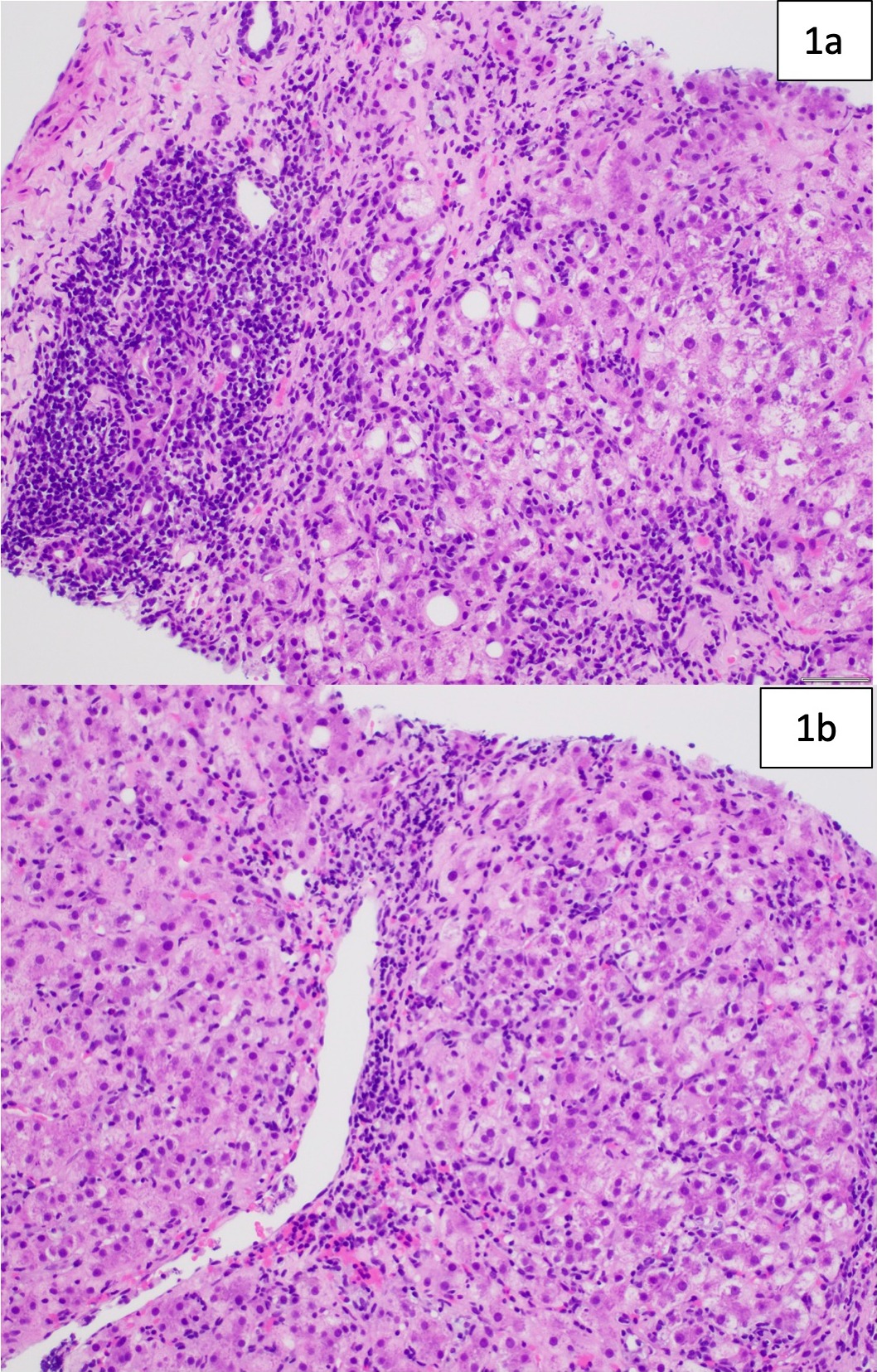
Figure: 1a: Image shows moderate portal-based chronic inflammation with conspicuous plasma cells (left side of the image) and at least moderate interface activity (right side of the image). Apoptotic hepatocytes are also identified. 1b: Inflammation rich in plasma cells, lymphocytes, and histiocytes extends throughout the lobule with areas of centrivenular accentuation. Increased fibrosis is also evident (not shown here). The overall findings are compatible with autoimmune hepatitis in the clinical context.
Disclosures:
Daniel Weinblatt indicated no relevant financial relationships.
Upasana Joneja indicated no relevant financial relationships.
Adib Chaaya indicated no relevant financial relationships.
Daniel Weinblatt, DO, Upasana Joneja, MD, Adib Chaaya, MD. D0525 - Autoimmune Hepatitis, SLE, and Leukocytoclastic Vasculitis Following the Moderna COVID Vaccine, ACG 2022 Annual Scientific Meeting Abstracts. Charlotte, NC: American College of Gastroenterology.
