Back

Poster Session D - Tuesday Morning
Category: Liver
D0611 - Acute Cholestatic Hepatitis Following m-RNABNT162b2 SARS-CoV-2 Vaccination: Could It Be Genetic?
Tuesday, October 25, 2022
10:00 AM – 12:00 PM ET
Location: Crown Ballroom

Has Audio

Melanie A. Hundt, MD
Los Angeles County + University of Southern California Medical Center; University of Southern California, Keck School of Medicine
Los Angeles, CA
Presenting Author(s)
Dana Toy, MD1, Melanie A. Hundt, MD2, Andrew Stolz, MD3
1University of Southern California Keck School of Medicine, Los Angeles, CA; 2Los Angeles County + University of Southern California Medical Center; University of Southern California, Keck School of Medicine, Los Angeles, CA; 3Los Angeles County + University of Southern California Medical Center, Los Angeles, CA
Introduction: Given the morbidity and mortality of COVID-19, development of vaccines targeting SARs-CoV-2 was essential. Despite their overall safety and efficacy, atypical complications have been reported. We report a case of post-vaccination acute cholestatic hepatitis, consistent with drug-induced liver injury (DILI).
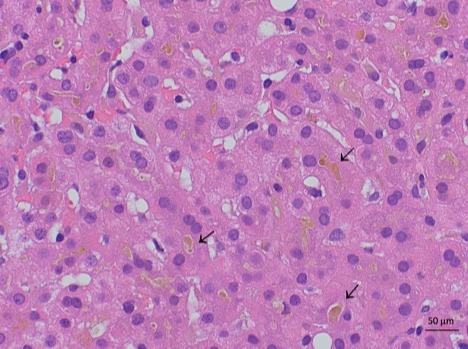
Case Description/Methods: A 61-year-old man with no past medical history presented with 2 weeks of jaundice and pruritus, 1 month after his second dose of the m-RNABNT162b2 SARS-CoV-2 vaccine. He denied use of any medications, supplements, tobacco, alcohol, drugs, or family history of liver disease. Vital signs and physical exam were unremarkable. Admission labs included ALP 265 U/L, AST 50 U/L, ALT 68 U/L, total bilirubin 14.9 mg/dL, and direct bilirubin 12 mg/dL. Ultrasound revealed normal liver surface without ductal dilatation, confirmed by MRCP. Serologies (HIV, HAV IgM, HBV core IgM, HBV surface antigen, HCV antibody), autoimmune markers (ANA, anti-mitochondrial, anti-actin, anti-liver-kidney-microsomal), and respiratory viral panel (SARS-CoV-2, influenza A/B, RSV) were negative. Hemoglobin, platelets, INR, albumin, gamma-glutamyl transpeptidase (GGT), and immunoglobulins were normal. Percutaneous liver biopsy revealed cholestasis with mild portal lymphocytic infiltrates and lobular inflammation (Figure 1), without portal fibrosis, PAS+ globules, or iron deposition. Molecular genetic testing (Next Generation Sequencing, Prevention Genetics® 77 Gene Cholestasis Panel) was performed. No genetic variants were identified in 77 genes associated with cholestasis, though single copy variants of several autosomal recessive genes were detected. Symptoms resolved with supportive care. Labs one month later were notable for ALP 175 U/L, (AST hemolyzed), ALT 68 U/L, and total bilirubin 3.2 mg/dL.
Discussion: Prior reports have described cholestasis due to immune-mediated hepatitis after COVID mRNA vaccination1, but these features were absent in this case. Given the patient’s normal GGT, we hypothesized that increased cytokine release led to cholestatic injury in an individual genetically predisposed to cholestasis by genetic variation in ATP8B1 or ABCB11. Future studies comparing genetic variants in patients with liver injury may provide insight into mechanisms of idiosyncratic DILI following mRNA vaccination against SARS-CoV-2.
Reference:
1. Bril F et al. Autoimmune hepatitis developing after coronavirus disease 2019 (COVID-19) vaccine: Casuality or casualty? J Hepatol. 2021 Jul; 75 (1): 222-224
Disclosures:
Dana Toy, MD1, Melanie A. Hundt, MD2, Andrew Stolz, MD3. D0611 - Acute Cholestatic Hepatitis Following m-RNABNT162b2 SARS-CoV-2 Vaccination: Could It Be Genetic?, ACG 2022 Annual Scientific Meeting Abstracts. Charlotte, NC: American College of Gastroenterology.
1University of Southern California Keck School of Medicine, Los Angeles, CA; 2Los Angeles County + University of Southern California Medical Center; University of Southern California, Keck School of Medicine, Los Angeles, CA; 3Los Angeles County + University of Southern California Medical Center, Los Angeles, CA
Introduction: Given the morbidity and mortality of COVID-19, development of vaccines targeting SARs-CoV-2 was essential. Despite their overall safety and efficacy, atypical complications have been reported. We report a case of post-vaccination acute cholestatic hepatitis, consistent with drug-induced liver injury (DILI).
Case Description/Methods: A 61-year-old man with no past medical history presented with 2 weeks of jaundice and pruritus, 1 month after his second dose of the m-RNABNT162b2 SARS-CoV-2 vaccine. He denied use of any medications, supplements, tobacco, alcohol, drugs, or family history of liver disease. Vital signs and physical exam were unremarkable. Admission labs included ALP 265 U/L, AST 50 U/L, ALT 68 U/L, total bilirubin 14.9 mg/dL, and direct bilirubin 12 mg/dL. Ultrasound revealed normal liver surface without ductal dilatation, confirmed by MRCP. Serologies (HIV, HAV IgM, HBV core IgM, HBV surface antigen, HCV antibody), autoimmune markers (ANA, anti-mitochondrial, anti-actin, anti-liver-kidney-microsomal), and respiratory viral panel (SARS-CoV-2, influenza A/B, RSV) were negative. Hemoglobin, platelets, INR, albumin, gamma-glutamyl transpeptidase (GGT), and immunoglobulins were normal. Percutaneous liver biopsy revealed cholestasis with mild portal lymphocytic infiltrates and lobular inflammation (Figure 1), without portal fibrosis, PAS+ globules, or iron deposition. Molecular genetic testing (Next Generation Sequencing, Prevention Genetics® 77 Gene Cholestasis Panel) was performed. No genetic variants were identified in 77 genes associated with cholestasis, though single copy variants of several autosomal recessive genes were detected. Symptoms resolved with supportive care. Labs one month later were notable for ALP 175 U/L, (AST hemolyzed), ALT 68 U/L, and total bilirubin 3.2 mg/dL.
Discussion: Prior reports have described cholestasis due to immune-mediated hepatitis after COVID mRNA vaccination1, but these features were absent in this case. Given the patient’s normal GGT, we hypothesized that increased cytokine release led to cholestatic injury in an individual genetically predisposed to cholestasis by genetic variation in ATP8B1 or ABCB11. Future studies comparing genetic variants in patients with liver injury may provide insight into mechanisms of idiosyncratic DILI following mRNA vaccination against SARS-CoV-2.
Reference:
1. Bril F et al. Autoimmune hepatitis developing after coronavirus disease 2019 (COVID-19) vaccine: Casuality or casualty? J Hepatol. 2021 Jul; 75 (1): 222-224

Figure: Liver biopsy showing cholestasis in a patient who developed jaundice after COVID mRNA vaccination
Disclosures:
Dana Toy indicated no relevant financial relationships.
Melanie Hundt indicated no relevant financial relationships.
Andrew Stolz indicated no relevant financial relationships.
Dana Toy, MD1, Melanie A. Hundt, MD2, Andrew Stolz, MD3. D0611 - Acute Cholestatic Hepatitis Following m-RNABNT162b2 SARS-CoV-2 Vaccination: Could It Be Genetic?, ACG 2022 Annual Scientific Meeting Abstracts. Charlotte, NC: American College of Gastroenterology.

