Back

Poster Session B - Monday Morning
Category: Liver
B0571 - A Case of Autoimmune Hepatitis in the Setting of the Janssen COVID-19 Vaccine
Monday, October 24, 2022
10:00 AM – 12:00 PM ET
Location: Crown Ballroom

Has Audio

Ahamed Khalyfa, DO
Franciscan Health
Olympia Fields, Illinois
Presenting Author(s)
Ahamed Khalyfa, DO1, Shil Punatar, DO2, Mohammad Arfeen, DO1, Tilemahos Spyratos, DO1
1Franciscan Health, Olympia Fields, IL; 2Franciscan Health Olympia Fields, Olympia Fields, IL
Introduction: Autoimmune hepatitis (AIH) is an inflammatory autoimmune condition that can result in chronic liver disease, it can be caused by the interaction of hereditary, epigenetic, immune, and environmental triggers. Vaccine related AIH is an uncommon phenomenon that has been reported in the presence of other vaccinations.. We present a unique case of AIH in the setting of the Janssen SARS-CoV-2 vaccine (JJ).
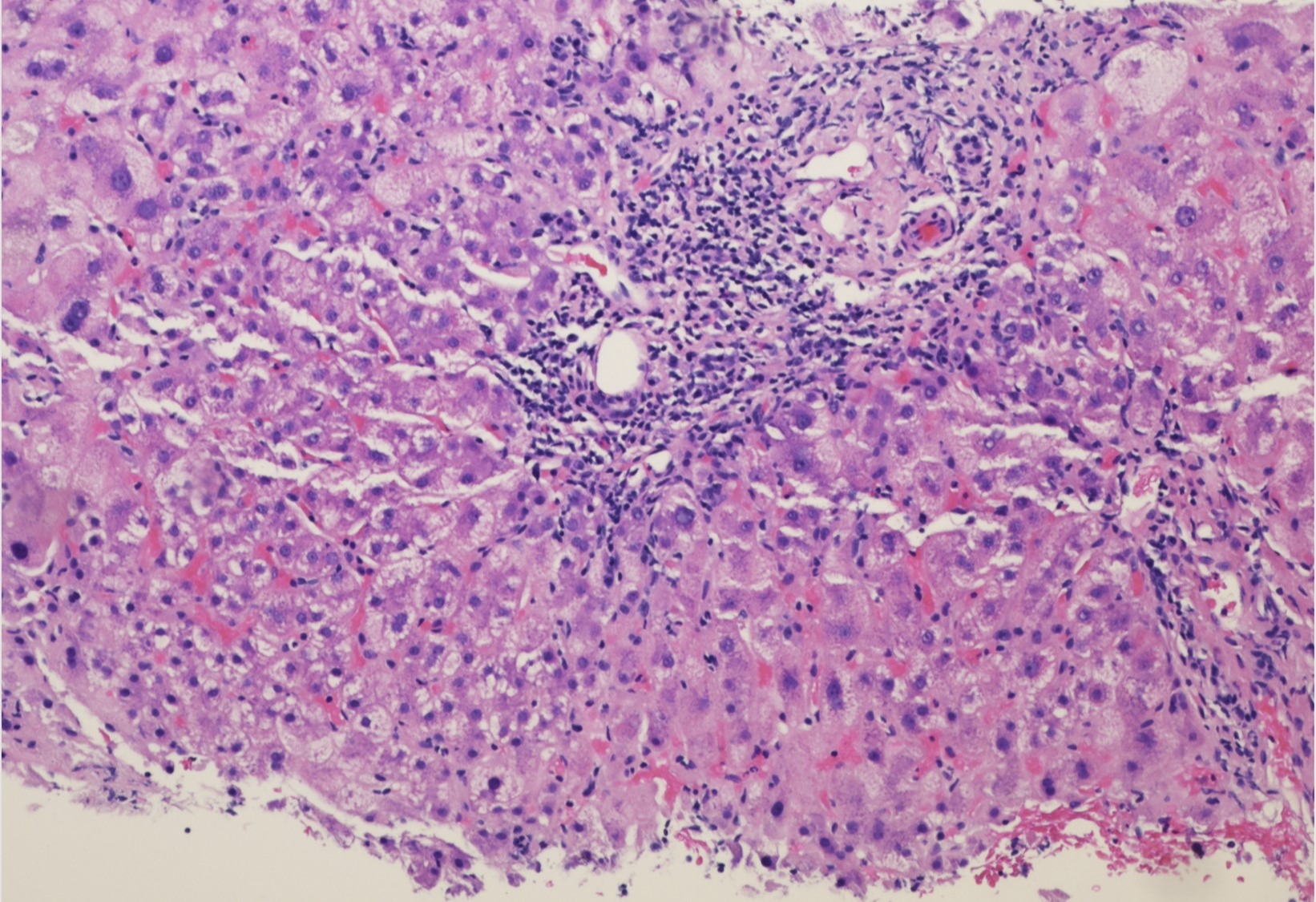
Case Description/Methods: A 45-year-old female with a history ofHTN, DM, GERD, and obesity presented from her primary care physician’s office with abnormal liver chemistries in a hepatocellular pattern. Her hepatic profile showed AST 1119, ALT 988, AP 130, and total bilirubin of 1.5 (R-factor 22.8). Given the magnitude and rapidity of the elevated chemistries a broad serologic workup was initiated. She was negative for hepatitis A, B, and C, as well as HSV 1/2, EBV, CMV, HIV, VZV, and adenovirus. Her ferritin and iron studies did not suggest iron overload. Her ceruloplasmin and alpha 1 antitrypsin levels were within acceptable ranges. Her ANA was negative; her smooth muscle antibody was positive (1:320), IgG was elevated to 3,451 mg/dL. Her urine toxin screen was negative. She had chronically been on HCTZ and took occasionally seamoss supplements. She denied using tylenol or any other over the counter or herbal supplements. In the past month she had received a single dose of the Janssen SARS CoV-2 vaccination (Johnson and Johnson). Her liver chemistries prior were AST 17, ALT 14, AP 52, and total bilirubin of 0.3. She subsequently underwent a liver biopsy which showed moderate (grade 3) lobular inflammatory activity, mild (grade 2) periportal activity, and mild (stage 2) fibrosis with an abundance of plasma cells consistent with autoimmune hepatitis. Treatment was initiated with oral prednisone with a resulting improvement in her liver chemistries.
Discussion: Autoimmune hepatitis in the setting of recent administration COVID 19 vaccine administration has been rarely reported. We present a unique case of COVID vaccine induced autoimmune hepatitis from a non-mRNA based vaccine. It has been suggested that possible mechanisms may include molecular mimicry or bystander activation of dormant autoreactive T-helper cells for both tissue-specific and non-tissue-specific reactions. We aim to shed light to this interesting paradigm and to spark the scientific discourse to further delineate potential mechanisms leading to this uncommon complication.
Disclosures:
Ahamed Khalyfa, DO1, Shil Punatar, DO2, Mohammad Arfeen, DO1, Tilemahos Spyratos, DO1. B0571 - A Case of Autoimmune Hepatitis in the Setting of the Janssen COVID-19 Vaccine, ACG 2022 Annual Scientific Meeting Abstracts. Charlotte, NC: American College of Gastroenterology.
1Franciscan Health, Olympia Fields, IL; 2Franciscan Health Olympia Fields, Olympia Fields, IL
Introduction: Autoimmune hepatitis (AIH) is an inflammatory autoimmune condition that can result in chronic liver disease, it can be caused by the interaction of hereditary, epigenetic, immune, and environmental triggers. Vaccine related AIH is an uncommon phenomenon that has been reported in the presence of other vaccinations.. We present a unique case of AIH in the setting of the Janssen SARS-CoV-2 vaccine (JJ).
Case Description/Methods: A 45-year-old female with a history ofHTN, DM, GERD, and obesity presented from her primary care physician’s office with abnormal liver chemistries in a hepatocellular pattern. Her hepatic profile showed AST 1119, ALT 988, AP 130, and total bilirubin of 1.5 (R-factor 22.8). Given the magnitude and rapidity of the elevated chemistries a broad serologic workup was initiated. She was negative for hepatitis A, B, and C, as well as HSV 1/2, EBV, CMV, HIV, VZV, and adenovirus. Her ferritin and iron studies did not suggest iron overload. Her ceruloplasmin and alpha 1 antitrypsin levels were within acceptable ranges. Her ANA was negative; her smooth muscle antibody was positive (1:320), IgG was elevated to 3,451 mg/dL. Her urine toxin screen was negative. She had chronically been on HCTZ and took occasionally seamoss supplements. She denied using tylenol or any other over the counter or herbal supplements. In the past month she had received a single dose of the Janssen SARS CoV-2 vaccination (Johnson and Johnson). Her liver chemistries prior were AST 17, ALT 14, AP 52, and total bilirubin of 0.3. She subsequently underwent a liver biopsy which showed moderate (grade 3) lobular inflammatory activity, mild (grade 2) periportal activity, and mild (stage 2) fibrosis with an abundance of plasma cells consistent with autoimmune hepatitis. Treatment was initiated with oral prednisone with a resulting improvement in her liver chemistries.
Discussion: Autoimmune hepatitis in the setting of recent administration COVID 19 vaccine administration has been rarely reported. We present a unique case of COVID vaccine induced autoimmune hepatitis from a non-mRNA based vaccine. It has been suggested that possible mechanisms may include molecular mimicry or bystander activation of dormant autoreactive T-helper cells for both tissue-specific and non-tissue-specific reactions. We aim to shed light to this interesting paradigm and to spark the scientific discourse to further delineate potential mechanisms leading to this uncommon complication.

Figure: Liver biopsy which showed moderate (grade 3) lobular inflammatory activity, mild (grade 2) periportal activity, and mild (stage 2) fibrosis with an abundance of plasma cells consistent with autoimmune hepatitis
Disclosures:
Ahamed Khalyfa indicated no relevant financial relationships.
Shil Punatar indicated no relevant financial relationships.
Mohammad Arfeen indicated no relevant financial relationships.
Tilemahos Spyratos indicated no relevant financial relationships.
Ahamed Khalyfa, DO1, Shil Punatar, DO2, Mohammad Arfeen, DO1, Tilemahos Spyratos, DO1. B0571 - A Case of Autoimmune Hepatitis in the Setting of the Janssen COVID-19 Vaccine, ACG 2022 Annual Scientific Meeting Abstracts. Charlotte, NC: American College of Gastroenterology.
