Back

Poster Session C - Monday Afternoon
Category: Liver
C0589 - Ezetimibe-Induced Autoimmune Hepatitis: An Uncommon Offender
Monday, October 24, 2022
3:00 PM – 5:00 PM ET
Location: Crown Ballroom

Has Audio

Anthony P. Nguyen, MD
University of New Mexico
Albuquerque, New Mexico
Presenting Author(s)
Anthony P. Nguyen, MD, Yiting Li, MD, Eyerusalem Akpan, MD, Christopher Chang, MD
University of New Mexico, Albuquerque, NM
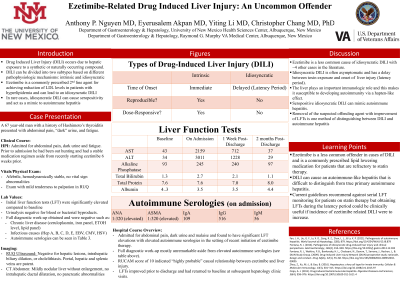
Introduction: Drug-induced autoimmune hepatitis (DIAIH) is a subset of autoimmune hepatitis (AIH) leading to a seropositive hepatitis caused by an inciting drug. Ezetimibe is a cholesterol absorption inhibitor within the intestinal tract that very rarely causes DIAIH. The proposed mechanism of Ezetimibe-induced DIAIH is thought to be due to interactions between drug metabolites and CYP450 within hepatic cells. Statins are another class of medication known to cause hepatotoxicity that are commonly used in combination with ezetimibe for hyperlipidemia. Highlighting cases of drug-related autoimmune hepatitis can be useful as routine liver function testing for statin-induced hepatitis is currently not recommended.
Case Description/Methods: This case describes a 67 year old man with hyperlipidemia and Hashimoto’s thyroiditis admitted for abdominal pain and biochemical evidence of hepatitis after starting ezetimibe 6 weeks prior. He had also been on atorvastatin but had been on a stable dose for the previous 7 years. He did not have a history of alcohol, herbal medication or Tylenol use and had entirely normal liver function tests 6 months prior to admission. Admission labs were pertinent for AST of 2159, ALT of 3011, Alkaline phosphatase of 245, total bilirubin of 2.7 (direct bilirubin 1.3), INR of 1.3, and a creatinine kinase of 497. Autoimmune serologies were notable for a positive ANA (1:320) and anti-smooth muscle antibody (1:320). An ultrasound was negative for hepatic lesions or evidence of advanced cirrhosis. Ezetimibe and atorvastatin therapy were discontinued on admission and the patient was eventually discharged after his liver function tests improved.
Discussion: Ezetimibe is a common 2nd line agent used for patients with statin-refractory hyperlipidemia which has also been known to cause hepatic injury typically with a latency period of 1-2 months into treatment. When used in combination with a statin the risk of hepatic injury increases. Current guidelines recommend against regular surveillance of liver function tests during statin treatment given that significant hepatotoxicity is exceedingly rare and routine testing did not prevent these events. However, in patients taking both a statin and ezetimibe it may be beneficial to obtain pre-treatment and in-treatment liver function testing towards the end of the latency period, especially if the incidence of ezetimibe-induced autoimmune hepatitis continues to rise.
Disclosures:
Anthony P. Nguyen, MD, Yiting Li, MD, Eyerusalem Akpan, MD, Christopher Chang, MD. C0589 - Ezetimibe-Induced Autoimmune Hepatitis: An Uncommon Offender, ACG 2022 Annual Scientific Meeting Abstracts. Charlotte, NC: American College of Gastroenterology.
University of New Mexico, Albuquerque, NM
Introduction: Drug-induced autoimmune hepatitis (DIAIH) is a subset of autoimmune hepatitis (AIH) leading to a seropositive hepatitis caused by an inciting drug. Ezetimibe is a cholesterol absorption inhibitor within the intestinal tract that very rarely causes DIAIH. The proposed mechanism of Ezetimibe-induced DIAIH is thought to be due to interactions between drug metabolites and CYP450 within hepatic cells. Statins are another class of medication known to cause hepatotoxicity that are commonly used in combination with ezetimibe for hyperlipidemia. Highlighting cases of drug-related autoimmune hepatitis can be useful as routine liver function testing for statin-induced hepatitis is currently not recommended.
Case Description/Methods: This case describes a 67 year old man with hyperlipidemia and Hashimoto’s thyroiditis admitted for abdominal pain and biochemical evidence of hepatitis after starting ezetimibe 6 weeks prior. He had also been on atorvastatin but had been on a stable dose for the previous 7 years. He did not have a history of alcohol, herbal medication or Tylenol use and had entirely normal liver function tests 6 months prior to admission. Admission labs were pertinent for AST of 2159, ALT of 3011, Alkaline phosphatase of 245, total bilirubin of 2.7 (direct bilirubin 1.3), INR of 1.3, and a creatinine kinase of 497. Autoimmune serologies were notable for a positive ANA (1:320) and anti-smooth muscle antibody (1:320). An ultrasound was negative for hepatic lesions or evidence of advanced cirrhosis. Ezetimibe and atorvastatin therapy were discontinued on admission and the patient was eventually discharged after his liver function tests improved.
Discussion: Ezetimibe is a common 2nd line agent used for patients with statin-refractory hyperlipidemia which has also been known to cause hepatic injury typically with a latency period of 1-2 months into treatment. When used in combination with a statin the risk of hepatic injury increases. Current guidelines recommend against regular surveillance of liver function tests during statin treatment given that significant hepatotoxicity is exceedingly rare and routine testing did not prevent these events. However, in patients taking both a statin and ezetimibe it may be beneficial to obtain pre-treatment and in-treatment liver function testing towards the end of the latency period, especially if the incidence of ezetimibe-induced autoimmune hepatitis continues to rise.
Disclosures:
Anthony Nguyen indicated no relevant financial relationships.
Yiting Li indicated no relevant financial relationships.
Eyerusalem Akpan indicated no relevant financial relationships.
Christopher Chang indicated no relevant financial relationships.
Anthony P. Nguyen, MD, Yiting Li, MD, Eyerusalem Akpan, MD, Christopher Chang, MD. C0589 - Ezetimibe-Induced Autoimmune Hepatitis: An Uncommon Offender, ACG 2022 Annual Scientific Meeting Abstracts. Charlotte, NC: American College of Gastroenterology.
