Back


Poster Session E - Tuesday Afternoon
Category: Colon
E0099 - Observations in Therapy Interventions for Stage III and Stage IV Colon Cancer From 2010 to 2019
Tuesday, October 25, 2022
3:00 PM – 5:00 PM ET
Location: Crown Ballroom

Has Audio

Monika Devanaboyina, BS
The University of Toledo, College of Medicine and Life Sciences
Toledo, Ohio
Presenting Author(s)
Monika Devanaboyina, BS1, Jordan Burlen, MD2
1The University of Toledo, College of Medicine and Life Sciences, Toledo, OH; 2The Ohio State University, Toledo, OH
Introduction: Colorectal cancer (CC) treatment options range from surgery, radiation therapy, systemic chemotherapy (CH), targeted immunotherapy (IM), or a combination of these. Surgical resection is the standard of care for cases with curative intent. CH is recommended in all cases of stage III cancer after surgery. Combination CH and IM have been FDA approved for use in recent years. With the advent of IM and the major breakthroughs in its curative ability, we intended to assess the shift in treatment of stage III and stage IV CC.
Methods: The 2022 National Cancer Database Public Benchmark reports from the American College of Surgeons from 1391 hospitals was utilized. IRB approval was not required as the database contains de-identified information. This study analyzed CC cases with first line course of treatment for AJCC stage III-IV. 15,897 patients in 2010 and 20,060 in 2019 with stage IV cancer were evaluated, respectively. For stage III CC, 20,036 and 21,954 were evaluated in 2010 and 2019, respectively. A two-sample proportion z-test for significance was used.
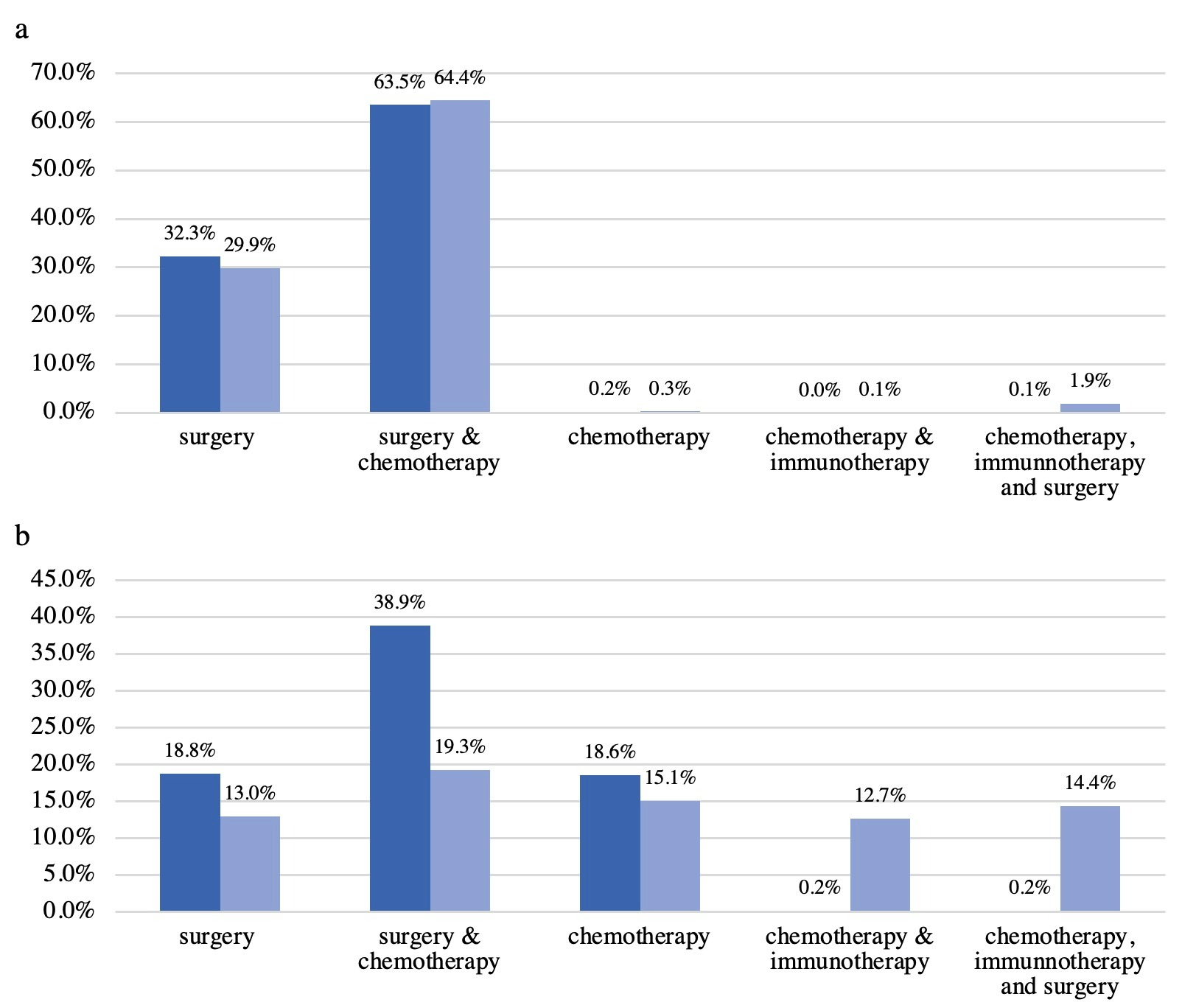
Results: Changes in trends were examined with stage III CC comparing the years 2010 and 2019 [Figure 1b]. Surgery in patients decreased (32.3% vs 29.9%, p< 0.001). Surgery in combination with CH did not change significantly (63.5% vs 64.4%, p= 0.006). CH only also did not change significantly (0.2% vs 0.3%, p= 0.039). IM combined with CH was limited (0% vs 0.1%). However, combination IM, surgery and CH increased use of IM (0.1% vs 1.9%, p< 0.001), and decreased treatment with surgery or CH alone.
CH alone was used in 18.6% of cases in 2010 compared to 15.1% in 2019 for stage IV CC (p< 0.001) [Figure 1b]. Surgical treatment decreased over the years from 18.8% to 13% (p< 0.001). Surgery and CH combination also decreased (38.9% vs 19.3%, p< 0.001). In contrast, treatment with IM and CH increased (0.2% vs. 12.7%, p< 0.001). IM in combination with surgery and CH also increased significantly in stage IV CC (0.2% vs 14.4%, p< 0.001).
Discussion: Treatment options between 2010 and 2019 were compared for stage III and stage IV CC. Stage IV CC treatment regimens have changed with increased use of IM and decreased sole use surgery and CH. However, stage III CC treatment regimens have largely been unchanged comparing 2010 to 2019. With the major successes of IM in treatment of early stage CC, there should be more work and studies to support its use in late stage.
Disclosures:
Monika Devanaboyina, BS1, Jordan Burlen, MD2. E0099 - Observations in Therapy Interventions for Stage III and Stage IV Colon Cancer From 2010 to 2019, ACG 2022 Annual Scientific Meeting Abstracts. Charlotte, NC: American College of Gastroenterology.
1The University of Toledo, College of Medicine and Life Sciences, Toledo, OH; 2The Ohio State University, Toledo, OH
Introduction: Colorectal cancer (CC) treatment options range from surgery, radiation therapy, systemic chemotherapy (CH), targeted immunotherapy (IM), or a combination of these. Surgical resection is the standard of care for cases with curative intent. CH is recommended in all cases of stage III cancer after surgery. Combination CH and IM have been FDA approved for use in recent years. With the advent of IM and the major breakthroughs in its curative ability, we intended to assess the shift in treatment of stage III and stage IV CC.
Methods: The 2022 National Cancer Database Public Benchmark reports from the American College of Surgeons from 1391 hospitals was utilized. IRB approval was not required as the database contains de-identified information. This study analyzed CC cases with first line course of treatment for AJCC stage III-IV. 15,897 patients in 2010 and 20,060 in 2019 with stage IV cancer were evaluated, respectively. For stage III CC, 20,036 and 21,954 were evaluated in 2010 and 2019, respectively. A two-sample proportion z-test for significance was used.
Results: Changes in trends were examined with stage III CC comparing the years 2010 and 2019 [Figure 1b]. Surgery in patients decreased (32.3% vs 29.9%, p< 0.001). Surgery in combination with CH did not change significantly (63.5% vs 64.4%, p= 0.006). CH only also did not change significantly (0.2% vs 0.3%, p= 0.039). IM combined with CH was limited (0% vs 0.1%). However, combination IM, surgery and CH increased use of IM (0.1% vs 1.9%, p< 0.001), and decreased treatment with surgery or CH alone.
CH alone was used in 18.6% of cases in 2010 compared to 15.1% in 2019 for stage IV CC (p< 0.001) [Figure 1b]. Surgical treatment decreased over the years from 18.8% to 13% (p< 0.001). Surgery and CH combination also decreased (38.9% vs 19.3%, p< 0.001). In contrast, treatment with IM and CH increased (0.2% vs. 12.7%, p< 0.001). IM in combination with surgery and CH also increased significantly in stage IV CC (0.2% vs 14.4%, p< 0.001).
Discussion: Treatment options between 2010 and 2019 were compared for stage III and stage IV CC. Stage IV CC treatment regimens have changed with increased use of IM and decreased sole use surgery and CH. However, stage III CC treatment regimens have largely been unchanged comparing 2010 to 2019. With the major successes of IM in treatment of early stage CC, there should be more work and studies to support its use in late stage.

Figure: Figure 1a. Stage III colorectal cancer treatment distribution for 2010 and 2019
Figure 1b. Stage IV colorectal cancer treatment distribution for 2010 and 2019
Figure 1b. Stage IV colorectal cancer treatment distribution for 2010 and 2019
Disclosures:
Monika Devanaboyina indicated no relevant financial relationships.
Jordan Burlen indicated no relevant financial relationships.
Monika Devanaboyina, BS1, Jordan Burlen, MD2. E0099 - Observations in Therapy Interventions for Stage III and Stage IV Colon Cancer From 2010 to 2019, ACG 2022 Annual Scientific Meeting Abstracts. Charlotte, NC: American College of Gastroenterology.
