Back

Poster Session E - Tuesday Afternoon
Category: Colon
E0122 - A Rare Case of BRAF/MEK Inhibitor-Induced Colitis
Tuesday, October 25, 2022
3:00 PM – 5:00 PM ET
Location: Crown Ballroom

Has Audio

Seyed Ali Khalessi Hosseini, MD
Rutgers New Jersey Medical School
Newark, New Jersey
Presenting Author(s)
Seyed Ali Khalessi Hosseini, MD1, Sally Fouda, MBBCh2, Ryan Stephenson, DO2, Lea Ann Chen, MD3
1Rutgers New Jersey Medical School, Newark, NJ; 2Rutgers Robert Wood Johnson, New Brunswick, NJ; 3Rutgers Robert Wood Johnson Medical School, New Brunswick, NJ
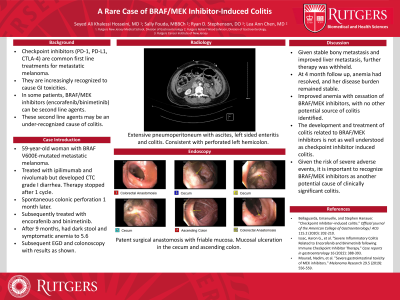
Introduction: Checkpoint inhibitors (e.g., PD-1, PD-L1, and CTLA-4) are first line treatments for metastatic melanoma but are increasingly recognized to cause GI toxicities, which may limit their use. We present a case of drug-induced colitis associated with the BRAF/MEK inhibitors encorafenib/binimetinib. These second line agents target mutated proteins in the MAP kinase signaling pathway and may be recommended for those who do not tolerate checkpoint inhibitors; however, they may also be a potential cause of colitis.
Case Description/Methods: A 59-year-old female with BRAF V600E-mutated metastatic melanoma was initially treated with ipilimumab (CTLA-4 inhibitor) and nivolumab (PD-L1 inhibitor). She developed CTC grade I diarrhea controlled with loperamide, but then also developed pathological fractures. Given disease progression and GI toxicity, therapy was stopped after 1 cycle. One month later, the patient experienced spontaneous colonic perforation attributed to her prior checkpoint inhibitors, necessitating left hemicolectomy and diverting ostomy. She was subsequently treated with encorafenib (BRAF inhibitor) and binimetinib (MEK inhibitor), but after 9 months developed dark stool, dyspnea, fatigue, and a hemoglobin of 5.6 g/dL from 10.2 g/dL one month prior. She had no diarrhea or other GI symptoms. Inpatient upper endoscopy revealed candida esophagitis. A colonoscopy found friable and ulcerated mucosa in the cecum and ascending colon. Pathology was notable for chronic inflammation of the lamina propria with eosinophilic infiltrates consistent with drug-induced colitis. The patient was treated with pRBC transfusions and fluconazole. Given her stable bony metastases and improved liver metastases, her BRAF and MEK inhibitors were withheld. At outpatient follow-up 4 months later, the anemia had resolved.
Discussion: Checkpoint inhibitors are well known causes of diarrhea and colitis. Patients with these complications and BRAF-mutated malignancies may be recommended to use BRAF and MEK inhibitors instead. However, the development and treatment of colitis related to these therapies are far less understood. Herein we present a patient who developed symptomatic anemia with no other potential source besides BRAF/MEK inhibitor induced colitis which improved after cessation of therapy. Given the risk of severe adverse events, it is important for clinicians to recognize that BRAF and MEK inhibitors may also cause clinically significant colitis.
Disclosures:
Seyed Ali Khalessi Hosseini, MD1, Sally Fouda, MBBCh2, Ryan Stephenson, DO2, Lea Ann Chen, MD3. E0122 - A Rare Case of BRAF/MEK Inhibitor-Induced Colitis, ACG 2022 Annual Scientific Meeting Abstracts. Charlotte, NC: American College of Gastroenterology.
1Rutgers New Jersey Medical School, Newark, NJ; 2Rutgers Robert Wood Johnson, New Brunswick, NJ; 3Rutgers Robert Wood Johnson Medical School, New Brunswick, NJ
Introduction: Checkpoint inhibitors (e.g., PD-1, PD-L1, and CTLA-4) are first line treatments for metastatic melanoma but are increasingly recognized to cause GI toxicities, which may limit their use. We present a case of drug-induced colitis associated with the BRAF/MEK inhibitors encorafenib/binimetinib. These second line agents target mutated proteins in the MAP kinase signaling pathway and may be recommended for those who do not tolerate checkpoint inhibitors; however, they may also be a potential cause of colitis.
Case Description/Methods: A 59-year-old female with BRAF V600E-mutated metastatic melanoma was initially treated with ipilimumab (CTLA-4 inhibitor) and nivolumab (PD-L1 inhibitor). She developed CTC grade I diarrhea controlled with loperamide, but then also developed pathological fractures. Given disease progression and GI toxicity, therapy was stopped after 1 cycle. One month later, the patient experienced spontaneous colonic perforation attributed to her prior checkpoint inhibitors, necessitating left hemicolectomy and diverting ostomy. She was subsequently treated with encorafenib (BRAF inhibitor) and binimetinib (MEK inhibitor), but after 9 months developed dark stool, dyspnea, fatigue, and a hemoglobin of 5.6 g/dL from 10.2 g/dL one month prior. She had no diarrhea or other GI symptoms. Inpatient upper endoscopy revealed candida esophagitis. A colonoscopy found friable and ulcerated mucosa in the cecum and ascending colon. Pathology was notable for chronic inflammation of the lamina propria with eosinophilic infiltrates consistent with drug-induced colitis. The patient was treated with pRBC transfusions and fluconazole. Given her stable bony metastases and improved liver metastases, her BRAF and MEK inhibitors were withheld. At outpatient follow-up 4 months later, the anemia had resolved.
Discussion: Checkpoint inhibitors are well known causes of diarrhea and colitis. Patients with these complications and BRAF-mutated malignancies may be recommended to use BRAF and MEK inhibitors instead. However, the development and treatment of colitis related to these therapies are far less understood. Herein we present a patient who developed symptomatic anemia with no other potential source besides BRAF/MEK inhibitor induced colitis which improved after cessation of therapy. Given the risk of severe adverse events, it is important for clinicians to recognize that BRAF and MEK inhibitors may also cause clinically significant colitis.
Disclosures:
Seyed Ali Khalessi Hosseini indicated no relevant financial relationships.
Sally Fouda indicated no relevant financial relationships.
Ryan Stephenson indicated no relevant financial relationships.
Lea Ann Chen: Intrinsic Medicine – Consultant. Phatom – Advisory Committee/Board Member. PredictImmune – Consultant, Grant/Research Support.
Seyed Ali Khalessi Hosseini, MD1, Sally Fouda, MBBCh2, Ryan Stephenson, DO2, Lea Ann Chen, MD3. E0122 - A Rare Case of BRAF/MEK Inhibitor-Induced Colitis, ACG 2022 Annual Scientific Meeting Abstracts. Charlotte, NC: American College of Gastroenterology.
