Back

Poster Session E - Tuesday Afternoon
Category: Colorectal Cancer Prevention
E0163 - Detection of Mutated Tumor DNA From Colorectal Cancer Using Real Time-PCR
Tuesday, October 25, 2022
3:00 PM – 5:00 PM ET
Location: Crown Ballroom

Has Audio

Erik Anderson, DO, PhD
SUNY Downstate Medical Center
Brooklyn, NY
Presenting Author(s)
Erik Anderson, DO, PhD1, Naveed Ahmed, DO1, Sofia C. Tortora, MS, DDS1, Laura Martello-Rooney, PhD1, Manuel Martinez, MD, FACG2
1SUNY Downstate Medical Center, Brooklyn, NY; 2Brooklyn VA NY Harbor Healthcare System, Brooklyn, NY
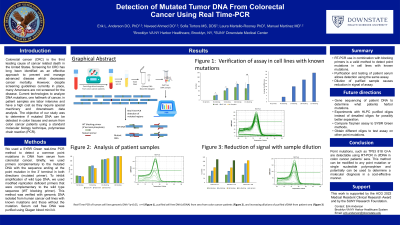
Introduction: Colorectal cancer (CRC) is the third leading cause of cancer related death in the United States. Screening for CRC has long been identified as an effective approach to prevent and manage advanced disease which decreases cancer mortality. However, despite screening guidelines currently in place, many Americans are not screened for the disease. Current technologies to analyze DNA mutations, one hallmark of cancer, in patient samples are labor intensive and have a high cost as they require special machinery and downstream data analysis. The objective of our study was to determine if mutated DNA can be detected in colon tissues and serum from CRC patients using a standard molecular biology technique, polymerase chain reaction (PCR).
Methods: We used a real-time PCR method to detect common point mutations in DNA in colorectal cancer (for example, TP53 818 G >A). Briefly, we used primers complementary to the mutated DNA with the sequence ending at the point mutation in the 3’ terminal in both directions (mutated primer). To inhibit amplification of wild type DNA, we used modified replication deficient primers that were complementary to the wild type sequence (WT blocking primer). This method was tested with genomic DNA isolated from human cancer cell lines with known mutations and those without the mutation.
Results: Using the PCR based approach with the cancer cell lines, we were able to obtain a cycle amplification (Cq) difference of up to 20, which correlates to a 10,000 fold amplification separation between the WT and mutated DNA.
Discussion: We tested a PCR based approach to detect the presence of mutated DNA which is relatively inexpensive compared to DNA sequencing. Primers used in this assay can be modified to detect any point mutations of interest in colorectal cancer. Further studies will determine and affirm the utility of detecting DNA mutations in the early diagnosis of colorectal cancer.
Disclosures:
Erik Anderson, DO, PhD1, Naveed Ahmed, DO1, Sofia C. Tortora, MS, DDS1, Laura Martello-Rooney, PhD1, Manuel Martinez, MD, FACG2. E0163 - Detection of Mutated Tumor DNA From Colorectal Cancer Using Real Time-PCR, ACG 2022 Annual Scientific Meeting Abstracts. Charlotte, NC: American College of Gastroenterology.
1SUNY Downstate Medical Center, Brooklyn, NY; 2Brooklyn VA NY Harbor Healthcare System, Brooklyn, NY
Introduction: Colorectal cancer (CRC) is the third leading cause of cancer related death in the United States. Screening for CRC has long been identified as an effective approach to prevent and manage advanced disease which decreases cancer mortality. However, despite screening guidelines currently in place, many Americans are not screened for the disease. Current technologies to analyze DNA mutations, one hallmark of cancer, in patient samples are labor intensive and have a high cost as they require special machinery and downstream data analysis. The objective of our study was to determine if mutated DNA can be detected in colon tissues and serum from CRC patients using a standard molecular biology technique, polymerase chain reaction (PCR).
Methods: We used a real-time PCR method to detect common point mutations in DNA in colorectal cancer (for example, TP53 818 G >A). Briefly, we used primers complementary to the mutated DNA with the sequence ending at the point mutation in the 3’ terminal in both directions (mutated primer). To inhibit amplification of wild type DNA, we used modified replication deficient primers that were complementary to the wild type sequence (WT blocking primer). This method was tested with genomic DNA isolated from human cancer cell lines with known mutations and those without the mutation.
Results: Using the PCR based approach with the cancer cell lines, we were able to obtain a cycle amplification (Cq) difference of up to 20, which correlates to a 10,000 fold amplification separation between the WT and mutated DNA.
Discussion: We tested a PCR based approach to detect the presence of mutated DNA which is relatively inexpensive compared to DNA sequencing. Primers used in this assay can be modified to detect any point mutations of interest in colorectal cancer. Further studies will determine and affirm the utility of detecting DNA mutations in the early diagnosis of colorectal cancer.
Disclosures:
Erik Anderson indicated no relevant financial relationships.
Naveed Ahmed indicated no relevant financial relationships.
Sofia Tortora indicated no relevant financial relationships.
Laura Martello-Rooney indicated no relevant financial relationships.
Manuel Martinez: Medtronic – Advisor or Review Panel Member. Pfizer – Stock-publicly held company(excluding mutual/index funds). Schlesinger Focus Group – Advisor or Review Panel Member.
Erik Anderson, DO, PhD1, Naveed Ahmed, DO1, Sofia C. Tortora, MS, DDS1, Laura Martello-Rooney, PhD1, Manuel Martinez, MD, FACG2. E0163 - Detection of Mutated Tumor DNA From Colorectal Cancer Using Real Time-PCR, ACG 2022 Annual Scientific Meeting Abstracts. Charlotte, NC: American College of Gastroenterology.
