Poster Session C - Monday Afternoon
Category: Functional Bowel Disease
C0281 - Symptomatic Response to Antibiotics in Patients With Small Intestine Bacterial Overgrowth: A Systematic Review and Meta-Analysis


Will Takakura, MD
Cedars Sinai - Medically Associated Science and Technology
Los Angeles, California
Presenting Author(s)
1Cedars Sinai - Medically Associated Science and Technology, Los Angeles, CA; 2Cedars Sinai - Medically Associated Science and Technology Program, Los Angeles, CA; 3Michigan Medicine, Ann Arbor, MI; 4Cedars-Sinai Medical Center, Los Angeles, CA
Introduction: Small intestinal bacterial overgrowth (SIBO) is characterized by an increase in proteobacteria in the small intestine and is associated with gastrointestinal symptoms. Antibiotics are used to treat this condition and previous meta-analyses have shown that antibiotics can successfully eradicate SIBO. Currently, there have been no high-quality, multicenter trials evaluating the efficacy of antibiotics in improving symptoms. Here, we performed a systematic review and meta-analysis to assess the efficacy of antibiotics to relive symptoms in patients with SIBO.
Methods: Following the PRISMA protocol a systematic review and meta-analysis was conducted. From inception to March 2021, MEDLINE, EMBASE, Web of Science, and Cochrane database were searched. Randomized controlled trials and prospective studies were included. Studies were included if SIBO was diagnosed based on breath test or small bowel aspirate. The rate of no improvement in symptoms were compared between antibiotics and no antibiotics. To assess study bias, the Cochrane Risk of Bias Tool was used. Studies with all categories with low risk of bias were deemed good quality. If 1 category had high or unclear risk of bias, they were deemed fair, the rest were deemed as poor quality.
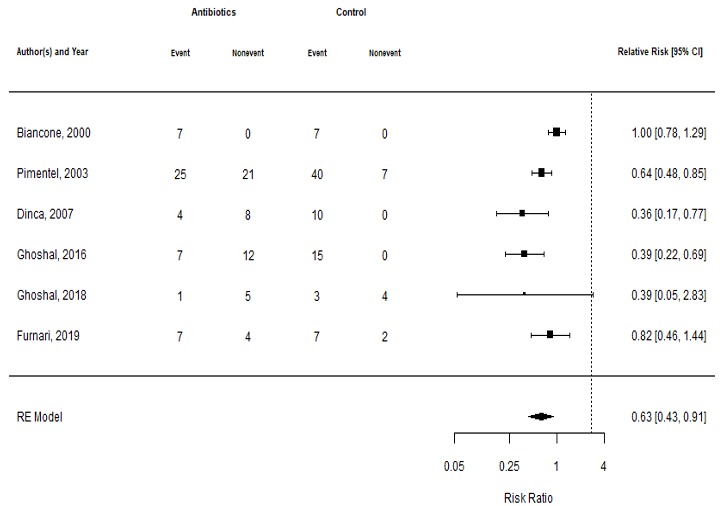
Results: Of 694 citations, 647 were excluded based on the title or abstract. After a full manuscript review, 6 studies met inclusion criteria and were included (Table 1). There was a total of 196 patients, out of which 101 received antibiotics and 95 received placebo or no antibiotics. Two studies compared rifaximin to placebo, 1 compared rifaximin with bran to placebo with bran, 1 compared norfloxacin to placebo, 1 compared neomycin to placebo, and another compared rifaximin to no antibiotics. Overall, the RR (95% CI) of no improvement was 0.63 (0.43-0.91) with antibiotics compared to no antibiotics or placebo (Figure 1). The NNT for antibiotics in relieving symptoms was 2.8. There was significant heterogeneity with I2 = 69.3%. There was no publication bias based on Egger’s test (t = -1.5936, df = 4, p = 0.1863). Four studies were deemed to be of poor quality and 2 studies were deemed to be of fair quality.
Discussion: This is the first systemic review and meta-analysis to evaluate the efficacy of antibiotics in relieving symptoms in patients with SIBO. Our data suggests that antibiotics provide symptomatic relief for patients with SIBO. A large multicenter randomized control trial is needed to validate these findings.
Study | Country | Sample size (% female) | Disease | Method to diagnose SIBO | Criteria for symptomatic improvement | Antibiotic used | Duration of therapy | Duration of follow-up | Rate of no improvement on antibiotics | Rate of no improvement on placebo |
Biancone 2000 | Italy | 14 (50%) | Crohn’s disease | Glucose breath test | Change in CDAI | Rifaximin 400 mg BID | 7 days | 7 days | 7/7 (100%) | 7/7 (100%) |
Pimentel 2003 | US | 93 (62%) | Rome I IBS | Lactulose breath test | ≥ 50% reduction in composite score of abdominal pain, diarrhea, and constipation | Neomycin 500 mg BID | 10 days | 7 days | 25/46 (54.3%) | 40/47 (85.1%) |
D’inca 2007 | Italy | 22 | Diverticular disease | Lactulose breath test | Global symptomatic improvement | Rifaximin 600 mg BID | 14 days | End of treatment | 4/12 (33.3%) | 10/10 (100%) |
Ghoshal 2016 | India | 34 (19%) | Rome III IBS | Duodenal aspirate 103 CFU | No longer meeting Rome III Criteria for IBS | Norfloxacin 400 mg BID | 10 days | 30 days | 7/19 (36.8%) | 15/15 (100%) |
Ghoshal 2018 | India | 13 (54%) | Rome III IBS-C or FC | Lactulose breath test (methane) | BSS ≥ 3 | Rifaximin 400 mg BID | 14 days | 7 days | 1/6 (16.7%) | 3/7 (42.9%) |
Furnari 2019 | Italy | 20 (48%) | Cystic Fibrosis | Glucose breath test | ≥ 50% reduction in composite GI score | Rifaximin 10 mg/kg TID (up to 400 mg TID) | 14 days | 21 days | 7/11 (63.6%) | 7/9 (77.8%) |
Disclosures:
Will Takakura, MD1, Jiajing Wang, PhD2, William D. Chey, MD3, Ali Rezaie, MD, MSc4, Mark Pimentel, MD1. C0281 - Symptomatic Response to Antibiotics in Patients With Small Intestine Bacterial Overgrowth: A Systematic Review and Meta-Analysis, ACG 2022 Annual Scientific Meeting Abstracts. Charlotte, NC: American College of Gastroenterology.
